11 Temmuz 2012 Çarşamba
10 Temmuz 2012 Salı
9 Temmuz 2012 Pazartesi
What's your Drinking Personality?
To contact us Click HERE
The BBC is running a fluffy piece on peoples drinking personalities. It seems a psychologist, Dr. Glenn Willson, has observed the behavior of 500 Brits while at bars and pubs and found that their body language belies their personality. The good doctor has determined that their are eight distinct "drinking personalities". No more, no less.
 From the article:
From the article:
Image via the BBC.
 From the article:
From the article:THE JACK-THE-LADSo, what's your drinking personality? I think I am clearly the "Ice Queen."This "peacock" is conscious of his image and will drink a bottled beer, or cider.
He is inclined to be confident and arrogant, and can be territorial in his gestures, spreading himself over as much space as possible, for example, pushing the glass well away from himself and leaning back in his chair.
If he is drinking with friends, he would be unlikely to welcome approaches from outside the group, unless sycophantic and ego-enhancing.
Image via the BBC.
Recent News Concerning HPV in Men, HPV is now linked to causing Throat Cancer
To contact us Click HERE
Throat Cancer Linked to HPV
By LAURA LANDRO
A sharp rise in a type of throat cancer among men is increasingly being linked to HPV, the sexually transmitted human papillomavirus that can cause cervical cancer in women.
A new study from the National Cancer Institute warns that if recent trends continue, the number of HPV-positive oral cancers among men could rise by nearly 30% by 2020. At that rate, it could surpass that of cervical cancers among women, which are expected to decline as a result of better screening.
The study is to be presented this week at the annual American Society of Clinical Oncology meeting.
Between 1988 and 2004, the researchers found, the incidence of HPV-positive oropharynx cancers—those that affect the back of the tongue and tonsil area—increased by 225%. Anil Chaturvedi, a National Cancer Institute investigator who led the research, estimates there were approximately 6,700 cases of HPV-positive oropharynx cancers in 2010, up from 4,000 to 4,500 in 2004, and cases are projected to increase 27% to 8,500 in 2020.
Find out more about HPV Currently, Merck markets the vaccine Gardisil
HPV Facts
Human papillomavirus is the most common sexually transmitted virus in the U.S.
More than half of sexually active men and women are infected with HPV at some time in their lives.
About 20 million Americans are currently infected and about six million more get infected each year.
HPV can cause cervical cancer in women, which is the second-leading cause of cancer deaths in women world-wide.
HPV is linked to a four- to five-fold increase in certain oral cancers, especially in men; about 25% of mouth and 35% of throat cancers are caused by HPV.
There are more than 100 different types of HPV virus. Some are low-risk while highrisk types can cause several cancers, including head and neck cancer, which is becoming more prevalent.
Source: Centers for Disease Control and Prevention; American Academy of Pediatrics
 Image via Wikipedia
Image via Wikipedia
Recent studies show about 25% of mouth and 35% of throat cancers are caused by HPV, according to the Centers for Disease Control and Prevention.
Men account for the majority of cases, and currently the highest prevalence is in men 40 to 55, says Eric Genden, chief of head and neck oncology at Mount Sinai Medical Center in New York. Studies have shown that the cancer can show up 10 years after exposure to HPV, which has become the most common sexually transmitted virus in the U.S.
"We are sitting at the cusp of a pandemic," says Dr. Genden.
Dr. Chaturvedi says more studies are needed to evaluate whether a vaccine now used to prevent HPV for genital warts and genital and anal cancers can prevent oral HPV infections.
The HPV vaccine, Gardasil, made by Merck & Co., was approved in 2006 for girls and young women up to age 26, but while it is routinely recommended, only about 27% of girls have received all three doses needed to confer protection.
The FDA in 2009 approved the vaccine for males ages 9 through 26 to reduce the risk of genital warts, and in 2010 approved it for both sexes for the prevention of anal cancers. However, the CDC has only a "permissive" recommendation for boys, rather than a routine recommendation, meaning doctors generally will only administer it if parents or patients ask for it, says Michael Brady, chairman of the American Academy of Pediatrics infectious disease committee.
Lauri Markowitz, a CDC medical epidemiologist, says the CDC advisory committee that sets vaccine recommendations will review new data related to the issue at a meeting next month. However, at present there aren't any clinical-trial data showing the effectiveness of the vaccine against oral infections, she says.
 Image via Wikipedia
Image via Wikipedia
A Merck spokeswoman says the company has no plans to study the potential of Gardasil to prevent these cancers.
Researchers say it isn't clear why men are at higher risk for HPV-positive oral cancers. But for both men and women a high lifetime number of sex partners is associated with the cancer.
Changes in sexual behaviors that include increased practice of oral sex are associated with the increase, but a 2007 New England Journal of Medicine article also said engagement in casual sex, early age at first intercourse, and infrequent use of condoms each were associated with HPV-positive oropharyngeal cancer. Mouth-to-mouth contact through kissing can't be ruled out as a transmission route.
Most infections don't cause symptoms and go away on their own. But HPV can cause genital warts and warts in the throat, and has been associated with vaginal, vulvar and anal cancers.
Anna Giuliano, chairwoman of the department of cancer epidemiology at the Moffitt Cancer Center in Tampa, Fla., who studies oral HPV infections of men in several countries, says the rise in cancers among men shows it is important for males, as well as girls, to be vaccinated.
Doctors typically don't test for HPV-positive oral cancers. But Jonathan Aviv, director of the voice and swallowing center at New York's ENT and Allergy Associates, says his group looks through a miniature camera inserted through the nose at the back of the throat and tongue, and can biopsy suspicious warts or tumors.
In addition to being asked about symptoms such as hoarseness, difficulty swallowing, a neck mass or mouth sore that won't heal, patients are asked to fill out a risk-assessment sheet that includes the number of lifetime oral-sex partners. "People do get upset sometimes, but if your sexual history puts you at an increased risk for HPV, you should go and see an ear, nose and throat doctor," says Dr. Aviv.
 Image via Wikipedia
Image via Wikipedia
Fortunately, says Mount Sinai's Dr. Genden, those with HPV-positive oral cancers have a disease survival rate of 85% to 90% over five years, higher than those with oral cancers that aren't linked to HPV, but are more commonly linked to alcohol use, tobacco, and radiation exposure.
Philip Keane, a 52-year-old photographer and partner in a marketing firm, found a lump on his neck while shaving, which was initially misdiagnosed as an infection. A scope of his throat showed it was HPV-positive throat cancer. Dr. Genden removed it last July using minimally invasive robotic surgery, and Mr. Keane had 6½ weeks of daily radiation after that, which left him cancer-free.
At first surprised and embarrassed, Mr. Keane says he has no reason to think he was at high risk; while he has young daughters who have been immunized, "I didn't know about HPV in men." He plans to have his 12-year-old son immunized as well.
May 31, 2011, 12:30 PM ET
A form of head and neck cancer associated with the sexually transmitted human papillomavirus is on the rise, especially in men, the WSJ reports.
Fast-rising rates of oropharyngeal cancer — tumors in the tonsil and back-of-the-tongue area — have been linked to changes in sexual behavior that include the increased practice of oral sex and a greater number of sexual partners.
But HPV-positive cancer has also been reported in individuals who report few or no sexual partners. It may also be possible for the virus to be transmitted to an infant via an infected mother’s birth canal. An HPV vaccine is routinely recommended for girls because the virus can cause cervical cancer.
The rise in HPV-positive head and neck cancers is leading to a new focus both on treatment of the disease, and whether recommending routine vaccination for boys could prevent oral infections and cancers. (A CDC advisory panel said in 2009 that it was fine for boys to get the vaccine, but recommended against routine administration.)
 Image via Wikipedia
Image via Wikipedia
Eric Genden, chief of head and neck oncology at Mt. Sinai Medical Center, tells the Health Blog that when treated appropriately, patients with HPV-positive cancers have an 85% to 90% disease-free survival rate over five years. By contrast, patients with HPV-negative head and neck cancers, which are often associated with smoking and drinking, typically have more advanced disease when the cancer is detected and face a five-year survival rate of only 25% to 40%, Genden says.
HPV-induced head and neck cancer responds well to almost all forms of cancer therapy including surgery, external beam radiotherapy and chemotherapy. At Mount Sinai, the use of robotic surgery and radiation –with no chemotherapy required — resulted in three-year survival rates of 90% and significantly improved quality of life for patients, its studies show. Robotic surgery is less invasive than non-robotic tumor surgeries, minimizing complications and recover time.
Philip Keane, a 52-year old photographer and father of three (pictured at upper right), had the surgery last July at Mount Sinai, followed by a six-and-a-half week course of radiation at Memorial Sloan-Kettering Cancer Center. Keane says he’s switched to a healthier diet, and didn’t develop many of the symptoms of radiation he was warned about, such as mouth or neck sores. He says that during his therapy, he was able to continue working and doing everything he did before he got sick, and is now cancer-free.
On the vaccine front, at a meeting next month the CDC plans to review data on the issue of the cost and benefits of routinely recommending HPV vaccination for boys.

Michael Brady, chair of the department of pediatrics at Nationwide Children’s Hospital in Columbus, Ohio and chairman of the infectious disease committee for the American Academy of Pediatrics, says the AAP is awaiting any updates before changing its own policies: routine recommendation for girls at age 11 to 12 (with catch-up for teens and young women who have not received the vaccine, up to age 26 years) and a permissive recommendation for boys — meaning families or teens can get it if they ask for it.
Brady explains that the gender discrepancy for HPV vaccine occurred because initial studies for safety and effectiveness were done in females and the idea was a high coverage rate in females would result in protection of heterosexual males via herd immunity.
But low coverage of females by the vaccine means that there is minimal protection of heterosexual males, and there’s increasing evidence of the impact of HPV-related genital, oral and anal cancers in men. There is also no value in a female vaccine program for homosexual men. “All [this leads] to a realization that males would benefit from the HPV vaccine,” Brady says.
The CDC’s own permissive recommendation for young men allowed for payment through the Vaccines for Children program, and a Merck spokeswoman says more commercial insurers are paying for the vaccine for males. But Brady says vaccines with that level of recommendation tend to not be discussed at physician visits, and administration of HPV vaccine to males has been very low.
Moreover, while clear evidence of a cost benefit in girls was shown prior to the release of the vaccine, cost/benefit data for males are still being determined. “The more that people look; the more that it is clear that males would benefit by receiving the HPV vaccine,” Brady says. “The discussion is [over] whether it merits a routine recommendation.”
Oral sex, HPV puts non-smoking men at highest risk for oral cancer: What are the facts?
BY LINDSAY GOLDWERT
DAILY NEWS STAFF WRITER
Monday, April 18, 2011
HPV, otherwise known as the human papillomavirus, is a leading cause of cervical cancer for women but the nasty virus is now causing a spike in oral cancer and ravaging an entirely different group: men.
Cases of oral cancer resulting from exposure to the HPV-16 strain of the virus are hitting epidemic proportions in the U.S., doctors say.
Though the mention of oral cancer evokes images of gravely-voiced chain-smokers, the disease now has a new face: mostly white, male, non-smokers in their late 30s and early 40s.
The tumors forming on the back of their tongues and tonsils have nothing to do with nicotine - they are directly linked to engaging in oral sex with multiple female partners.
"If you've had more than five or six sexual partners, you are at a higher risk," Dr. Eric M. Genden, professor and chair of head and neck surgery at Mount Sinai Medical center told the Daily News. "We're only now beginning to see the beginning of a bell curve."
Women can get it from men as well although their chances are lower, according to doctors.
The human papillomavirus (HPV), a nasty bug with strains that causes genital warts and cervical cancer in women, is now the top cause of oral cancer in men, beating out smoking and drinking, according to reports from the New England Journal of Medicine and the Journal of Oncology as well as other research and treating institutions.
The number of smokers in the U.S. has steadily declined in the past 50 years, yet the rate of oral cancer has remained relatively steady and recently been on the increase.
The reason is an increase in HPV-16 in the U.S. population.
 Oral cancer is frightening because it often goes undetected until a lump appears on the neck or a patient begins to suffer from a persistent hoarseness. By then, even though the patient may feel fine, he could be in the late stages of the deadly disease.
Oral cancer is frightening because it often goes undetected until a lump appears on the neck or a patient begins to suffer from a persistent hoarseness. By then, even though the patient may feel fine, he could be in the late stages of the deadly disease.
If detected early, the chance for surviving oral cancer from HPV is high, between 85 to 90%. Treatments - localized radiation, chemotherapy and sometimes surgery - are effective when used in the early stages. When surgery is part of the solution, the options can be conventional surgery or a new robotic surgical technique, which reduces scarring and side effects in some patients.
Brian Hill, a medical device manufacturer, was in his mid-forties when found a hard spot on his neck 14 years ago. He went to his ear, nose and throat doctor, who dismissed the bump as an infection and prescribed antibiotics.
But a biopsy later found the lump was cancerous.
Though he had never smoked a cigarette, Hill was told he had a low chance of survival as the node in his neck was positive for squamous cell carcinoma, the most common oral cancer.
Hill has since founded the Oral Cancer Foundation and spends his time working with patients, doctors and dentists, as well as campaigning in Washington to raise awareness about the danger of oral cancer and the links to HPV.
"Anyone old enough to have engaged in sexual behaviors known to transmit this virus needs to be screened annually for oral cancer," said Hill. "It's the only way to catch this disease at its early stages."
Hill believes many doctors and researchers are still in the dark about the ties between HPV-16 and oral cancer, despite the rise in diagnoses in the past decade.
"We don't know exactly if or how long HPV-16 may lay dormant, or why it strikes certain people and not others," he said. "What we don't know exceeds what we do know."
Nearly all sexually active Americans will come in contact with HPV, according to the Oral Cancer Foundation and the National Institutes of Health.
Young women are encouraged to get the HPV vaccine, but doctors say there is little point in vaccinating patients past the age of 26 since the likelihood of prior exposure to cancer-causing strains is so high.
Young people now are having a lot more oral sex with many more partners so exposure rates are higher.
HPV is the most common STD in the U.S. About 20 million Americans are currently infected with HPV. Another 6 million people become newly infected each year, according to the Centers for Disease Control and Prevention.
HPV is passed through skin-to-skin contact, not fluids. Besides conventional sex, oral sex and even deep French kissing may spread the virus.
Doctors are sounding the alarm, but have stopped short of advising men to abstain.
"This is not a call to stop having oral sex," said Dr. Mark D. DeLacure, a head and neck surgeon at NYU's Langone Medical Center. "People have to continue living their lives, however we make the best choices when we know all the risks."
DeLacure said using condoms and dental dams during oral sex could reduce transmission, but acknowledges the idea is hardly appealing or practical.
"Limiting your sexual partners is a way to reduce your risk," said DeLacure. "But still, there are no guarantees."
Doctors also have this advice: Don't panic.
Patients with HPV - even the cancer-causing strain - may never develop cancer and may never transmit the virus to a partner.
The vast majority of individuals have immune systems that recognize the virus as a threat and easily defeat it.
Doctors instead recommend vigilance.
The important issue is persistence - meaning attention to how long an unusual symptom like a lump has lasted. When an abnormality persists for longer than 14 to 21 days, it's time to see an expert.
"If you've got a sore tonsil that is still a problem after a couple of weeks and particularly if it's localized to one side, I would say that's sign it's time to talk to a doctor," said DeLacure.
And talk to the person who sees your mouth the most: Your dentist.
Dentists, too, are becoming more aware of HPV and its role in the development of oral cancer.
A good dentist will know what to look for and where to look for it.
 Image via Wikipedia
Image via Wikipedia
Related articles

By LAURA LANDRO
A sharp rise in a type of throat cancer among men is increasingly being linked to HPV, the sexually transmitted human papillomavirus that can cause cervical cancer in women.
A new study from the National Cancer Institute warns that if recent trends continue, the number of HPV-positive oral cancers among men could rise by nearly 30% by 2020. At that rate, it could surpass that of cervical cancers among women, which are expected to decline as a result of better screening.
The study is to be presented this week at the annual American Society of Clinical Oncology meeting.
Between 1988 and 2004, the researchers found, the incidence of HPV-positive oropharynx cancers—those that affect the back of the tongue and tonsil area—increased by 225%. Anil Chaturvedi, a National Cancer Institute investigator who led the research, estimates there were approximately 6,700 cases of HPV-positive oropharynx cancers in 2010, up from 4,000 to 4,500 in 2004, and cases are projected to increase 27% to 8,500 in 2020.
Find out more about HPV Currently, Merck markets the vaccine Gardisil
HPV Facts
Human papillomavirus is the most common sexually transmitted virus in the U.S.
More than half of sexually active men and women are infected with HPV at some time in their lives.
About 20 million Americans are currently infected and about six million more get infected each year.
HPV can cause cervical cancer in women, which is the second-leading cause of cancer deaths in women world-wide.
HPV is linked to a four- to five-fold increase in certain oral cancers, especially in men; about 25% of mouth and 35% of throat cancers are caused by HPV.
There are more than 100 different types of HPV virus. Some are low-risk while highrisk types can cause several cancers, including head and neck cancer, which is becoming more prevalent.
Source: Centers for Disease Control and Prevention; American Academy of Pediatrics
 Image via Wikipedia
Image via WikipediaRecent studies show about 25% of mouth and 35% of throat cancers are caused by HPV, according to the Centers for Disease Control and Prevention.
Men account for the majority of cases, and currently the highest prevalence is in men 40 to 55, says Eric Genden, chief of head and neck oncology at Mount Sinai Medical Center in New York. Studies have shown that the cancer can show up 10 years after exposure to HPV, which has become the most common sexually transmitted virus in the U.S.
"We are sitting at the cusp of a pandemic," says Dr. Genden.
Dr. Chaturvedi says more studies are needed to evaluate whether a vaccine now used to prevent HPV for genital warts and genital and anal cancers can prevent oral HPV infections.
The HPV vaccine, Gardasil, made by Merck & Co., was approved in 2006 for girls and young women up to age 26, but while it is routinely recommended, only about 27% of girls have received all three doses needed to confer protection.
The FDA in 2009 approved the vaccine for males ages 9 through 26 to reduce the risk of genital warts, and in 2010 approved it for both sexes for the prevention of anal cancers. However, the CDC has only a "permissive" recommendation for boys, rather than a routine recommendation, meaning doctors generally will only administer it if parents or patients ask for it, says Michael Brady, chairman of the American Academy of Pediatrics infectious disease committee.
Lauri Markowitz, a CDC medical epidemiologist, says the CDC advisory committee that sets vaccine recommendations will review new data related to the issue at a meeting next month. However, at present there aren't any clinical-trial data showing the effectiveness of the vaccine against oral infections, she says.
 Image via Wikipedia
Image via WikipediaA Merck spokeswoman says the company has no plans to study the potential of Gardasil to prevent these cancers.
Researchers say it isn't clear why men are at higher risk for HPV-positive oral cancers. But for both men and women a high lifetime number of sex partners is associated with the cancer.
Changes in sexual behaviors that include increased practice of oral sex are associated with the increase, but a 2007 New England Journal of Medicine article also said engagement in casual sex, early age at first intercourse, and infrequent use of condoms each were associated with HPV-positive oropharyngeal cancer. Mouth-to-mouth contact through kissing can't be ruled out as a transmission route.
Most infections don't cause symptoms and go away on their own. But HPV can cause genital warts and warts in the throat, and has been associated with vaginal, vulvar and anal cancers.
Anna Giuliano, chairwoman of the department of cancer epidemiology at the Moffitt Cancer Center in Tampa, Fla., who studies oral HPV infections of men in several countries, says the rise in cancers among men shows it is important for males, as well as girls, to be vaccinated.
Doctors typically don't test for HPV-positive oral cancers. But Jonathan Aviv, director of the voice and swallowing center at New York's ENT and Allergy Associates, says his group looks through a miniature camera inserted through the nose at the back of the throat and tongue, and can biopsy suspicious warts or tumors.
In addition to being asked about symptoms such as hoarseness, difficulty swallowing, a neck mass or mouth sore that won't heal, patients are asked to fill out a risk-assessment sheet that includes the number of lifetime oral-sex partners. "People do get upset sometimes, but if your sexual history puts you at an increased risk for HPV, you should go and see an ear, nose and throat doctor," says Dr. Aviv.
 Image via Wikipedia
Image via WikipediaFortunately, says Mount Sinai's Dr. Genden, those with HPV-positive oral cancers have a disease survival rate of 85% to 90% over five years, higher than those with oral cancers that aren't linked to HPV, but are more commonly linked to alcohol use, tobacco, and radiation exposure.
Philip Keane, a 52-year-old photographer and partner in a marketing firm, found a lump on his neck while shaving, which was initially misdiagnosed as an infection. A scope of his throat showed it was HPV-positive throat cancer. Dr. Genden removed it last July using minimally invasive robotic surgery, and Mr. Keane had 6½ weeks of daily radiation after that, which left him cancer-free.
At first surprised and embarrassed, Mr. Keane says he has no reason to think he was at high risk; while he has young daughters who have been immunized, "I didn't know about HPV in men." He plans to have his 12-year-old son immunized as well.
May 31, 2011, 12:30 PM ET
With HPV-Related Head and Neck Cancers Rising, Focus on Treatment and Vaccination
By Laura LandroA form of head and neck cancer associated with the sexually transmitted human papillomavirus is on the rise, especially in men, the WSJ reports.
Fast-rising rates of oropharyngeal cancer — tumors in the tonsil and back-of-the-tongue area — have been linked to changes in sexual behavior that include the increased practice of oral sex and a greater number of sexual partners.
But HPV-positive cancer has also been reported in individuals who report few or no sexual partners. It may also be possible for the virus to be transmitted to an infant via an infected mother’s birth canal. An HPV vaccine is routinely recommended for girls because the virus can cause cervical cancer.
The rise in HPV-positive head and neck cancers is leading to a new focus both on treatment of the disease, and whether recommending routine vaccination for boys could prevent oral infections and cancers. (A CDC advisory panel said in 2009 that it was fine for boys to get the vaccine, but recommended against routine administration.)
 Image via Wikipedia
Image via WikipediaEric Genden, chief of head and neck oncology at Mt. Sinai Medical Center, tells the Health Blog that when treated appropriately, patients with HPV-positive cancers have an 85% to 90% disease-free survival rate over five years. By contrast, patients with HPV-negative head and neck cancers, which are often associated with smoking and drinking, typically have more advanced disease when the cancer is detected and face a five-year survival rate of only 25% to 40%, Genden says.
HPV-induced head and neck cancer responds well to almost all forms of cancer therapy including surgery, external beam radiotherapy and chemotherapy. At Mount Sinai, the use of robotic surgery and radiation –with no chemotherapy required — resulted in three-year survival rates of 90% and significantly improved quality of life for patients, its studies show. Robotic surgery is less invasive than non-robotic tumor surgeries, minimizing complications and recover time.
Philip Keane, a 52-year old photographer and father of three (pictured at upper right), had the surgery last July at Mount Sinai, followed by a six-and-a-half week course of radiation at Memorial Sloan-Kettering Cancer Center. Keane says he’s switched to a healthier diet, and didn’t develop many of the symptoms of radiation he was warned about, such as mouth or neck sores. He says that during his therapy, he was able to continue working and doing everything he did before he got sick, and is now cancer-free.
On the vaccine front, at a meeting next month the CDC plans to review data on the issue of the cost and benefits of routinely recommending HPV vaccination for boys.

Michael Brady, chair of the department of pediatrics at Nationwide Children’s Hospital in Columbus, Ohio and chairman of the infectious disease committee for the American Academy of Pediatrics, says the AAP is awaiting any updates before changing its own policies: routine recommendation for girls at age 11 to 12 (with catch-up for teens and young women who have not received the vaccine, up to age 26 years) and a permissive recommendation for boys — meaning families or teens can get it if they ask for it.
Brady explains that the gender discrepancy for HPV vaccine occurred because initial studies for safety and effectiveness were done in females and the idea was a high coverage rate in females would result in protection of heterosexual males via herd immunity.
But low coverage of females by the vaccine means that there is minimal protection of heterosexual males, and there’s increasing evidence of the impact of HPV-related genital, oral and anal cancers in men. There is also no value in a female vaccine program for homosexual men. “All [this leads] to a realization that males would benefit from the HPV vaccine,” Brady says.
The CDC’s own permissive recommendation for young men allowed for payment through the Vaccines for Children program, and a Merck spokeswoman says more commercial insurers are paying for the vaccine for males. But Brady says vaccines with that level of recommendation tend to not be discussed at physician visits, and administration of HPV vaccine to males has been very low.
Moreover, while clear evidence of a cost benefit in girls was shown prior to the release of the vaccine, cost/benefit data for males are still being determined. “The more that people look; the more that it is clear that males would benefit by receiving the HPV vaccine,” Brady says. “The discussion is [over] whether it merits a routine recommendation.”
Oral sex, HPV puts non-smoking men at highest risk for oral cancer: What are the facts?
BY LINDSAY GOLDWERT
DAILY NEWS STAFF WRITER
Monday, April 18, 2011
HPV, otherwise known as the human papillomavirus, is a leading cause of cervical cancer for women but the nasty virus is now causing a spike in oral cancer and ravaging an entirely different group: men.
Cases of oral cancer resulting from exposure to the HPV-16 strain of the virus are hitting epidemic proportions in the U.S., doctors say.
Though the mention of oral cancer evokes images of gravely-voiced chain-smokers, the disease now has a new face: mostly white, male, non-smokers in their late 30s and early 40s.
The tumors forming on the back of their tongues and tonsils have nothing to do with nicotine - they are directly linked to engaging in oral sex with multiple female partners.
"If you've had more than five or six sexual partners, you are at a higher risk," Dr. Eric M. Genden, professor and chair of head and neck surgery at Mount Sinai Medical center told the Daily News. "We're only now beginning to see the beginning of a bell curve."
Women can get it from men as well although their chances are lower, according to doctors.
The human papillomavirus (HPV), a nasty bug with strains that causes genital warts and cervical cancer in women, is now the top cause of oral cancer in men, beating out smoking and drinking, according to reports from the New England Journal of Medicine and the Journal of Oncology as well as other research and treating institutions.
The number of smokers in the U.S. has steadily declined in the past 50 years, yet the rate of oral cancer has remained relatively steady and recently been on the increase.
The reason is an increase in HPV-16 in the U.S. population.
 Oral cancer is frightening because it often goes undetected until a lump appears on the neck or a patient begins to suffer from a persistent hoarseness. By then, even though the patient may feel fine, he could be in the late stages of the deadly disease.
Oral cancer is frightening because it often goes undetected until a lump appears on the neck or a patient begins to suffer from a persistent hoarseness. By then, even though the patient may feel fine, he could be in the late stages of the deadly disease.If detected early, the chance for surviving oral cancer from HPV is high, between 85 to 90%. Treatments - localized radiation, chemotherapy and sometimes surgery - are effective when used in the early stages. When surgery is part of the solution, the options can be conventional surgery or a new robotic surgical technique, which reduces scarring and side effects in some patients.
Brian Hill, a medical device manufacturer, was in his mid-forties when found a hard spot on his neck 14 years ago. He went to his ear, nose and throat doctor, who dismissed the bump as an infection and prescribed antibiotics.
But a biopsy later found the lump was cancerous.
Though he had never smoked a cigarette, Hill was told he had a low chance of survival as the node in his neck was positive for squamous cell carcinoma, the most common oral cancer.
Hill has since founded the Oral Cancer Foundation and spends his time working with patients, doctors and dentists, as well as campaigning in Washington to raise awareness about the danger of oral cancer and the links to HPV.
"Anyone old enough to have engaged in sexual behaviors known to transmit this virus needs to be screened annually for oral cancer," said Hill. "It's the only way to catch this disease at its early stages."
Hill believes many doctors and researchers are still in the dark about the ties between HPV-16 and oral cancer, despite the rise in diagnoses in the past decade.
"We don't know exactly if or how long HPV-16 may lay dormant, or why it strikes certain people and not others," he said. "What we don't know exceeds what we do know."
Nearly all sexually active Americans will come in contact with HPV, according to the Oral Cancer Foundation and the National Institutes of Health.
Young women are encouraged to get the HPV vaccine, but doctors say there is little point in vaccinating patients past the age of 26 since the likelihood of prior exposure to cancer-causing strains is so high.
Young people now are having a lot more oral sex with many more partners so exposure rates are higher.
HPV is the most common STD in the U.S. About 20 million Americans are currently infected with HPV. Another 6 million people become newly infected each year, according to the Centers for Disease Control and Prevention.
HPV is passed through skin-to-skin contact, not fluids. Besides conventional sex, oral sex and even deep French kissing may spread the virus.
Doctors are sounding the alarm, but have stopped short of advising men to abstain.
"This is not a call to stop having oral sex," said Dr. Mark D. DeLacure, a head and neck surgeon at NYU's Langone Medical Center. "People have to continue living their lives, however we make the best choices when we know all the risks."
DeLacure said using condoms and dental dams during oral sex could reduce transmission, but acknowledges the idea is hardly appealing or practical.
"Limiting your sexual partners is a way to reduce your risk," said DeLacure. "But still, there are no guarantees."
Doctors also have this advice: Don't panic.
Patients with HPV - even the cancer-causing strain - may never develop cancer and may never transmit the virus to a partner.
The vast majority of individuals have immune systems that recognize the virus as a threat and easily defeat it.
Doctors instead recommend vigilance.
The important issue is persistence - meaning attention to how long an unusual symptom like a lump has lasted. When an abnormality persists for longer than 14 to 21 days, it's time to see an expert.
"If you've got a sore tonsil that is still a problem after a couple of weeks and particularly if it's localized to one side, I would say that's sign it's time to talk to a doctor," said DeLacure.
And talk to the person who sees your mouth the most: Your dentist.
Dentists, too, are becoming more aware of HPV and its role in the development of oral cancer.
A good dentist will know what to look for and where to look for it.
 Image via Wikipedia
Image via WikipediaRelated articles
- Significant Rise in HPV-related Throat Cancer in Men. (abcnews.go.com)

GEN | Analysis & Insight: Cloud Computing Augments Clinical Trial Process
To contact us Click HERE
GEN | Analysis & Insight: Cloud Computing Augments Clinical Trial Process
(Page 1 of 1)
(Page 1 of 1)
- Pharma and biotech companies have so far tended to use cloud computing services for drug development research, not clinical trials. IBM and other smaller tech companies would like to change all that, though.Additionally, applying cloud computing for data analysis to aid patient care is catching on. As cloud computing extends to clinical data analysis, which would be considered private information, security will loom larger as an issue for drug development companies.The benefits of using the cloud to store, manage, and analyze clinical data are similar to those for other drug development work. The cloud offers time on gigantic, dispersed infrastructures on a pay-as-you-go basis. It is estimated that 8–12% of clinical trial costs come from the need to move data around among various trial sites, comparing the data as it is being created, according to Erich Clementi, vp of strategy and GM of enterprise initiatives at IBM.Additionally, for projects that require heavy data crunching, cloud computing enables vast amounts of processing at a lower cost. For example, Jeffrey T. Leek, Ph.D., assistant professor at Johns Hopkins Bloomberg School of Public Health, and colleagues used an internally developed, open-source cloud-computing platform called Myrna for calculating differential gene expression in large RNA sequencing datasets.Running an analysis for a single RNA sequence on one laptop could take up to three weeks to complete, Dr. Leek explained. In contrast, by renting computers for cloud computation services for $65, Dr. Leek said he could get results back in an hour and 45 minutes. Continued....
Private Data Means Need for Security
One component of using cloud computing resources is the extent to which it can be “anonymized,” Dr. Leek told GEN. “For most of our analyses we have used publicly available data. There are a host of privacy issues that will come into play once clinical patient data comes into the picture. It will be a matter for the FDA, NIH, and institutional review boards to consider when evaluating projects.”Mika Nuutilainen, director, product development at CRF Health, in an interview with GEN said, “For clinical trial data management, I do not yet see public clouds as a viable solution. The current acceptable way of proving correct installation and operation of clinical trial data management systems is still very traditional and highly focused on hardware units and their location.” CRF Health provides technology for ePRO (electronic patient reported outcomes), a method used in clinical trials to collect information on a patient’s health status directly from the patient.Nuutilainen predicts that “private clouds” will be used, but added, “that is a solution where a dedicated server environment is boosted with automatic management software to allow, for example, fast disaster recovery from one site to another. ‘Community clouds’ could be used if clinical companies can co-operate that much and there is a technology-oriented company driving the development.“I personally do not consider the concept of ‘private clouds’ as cloud computing, instead I consider it a ‘virtualized environment.’ You still need to buy, install, and operate your environment, so a majority of the benefits from cloud computing is not achieved,” he pointed out.Clinical Trial Cloud Solutions
Stuart Henderson, IBM’s Americas life sciences R&D leader, believes that cloud computing for clinical trials has unique advantages. IBM offers “Clinical Cloud” to the drug development industry. The aim was to create a secure environment where partners can work together with quicker and easier access to data.The company said that the Clinical Cloud has the requisite features for an “intelligent” platform: multitenant security, infrastructure, process integration and orchestration, a clinical application suite, compliance, analytics, collaboration, help and support, data sharing, and file sharing.Multiple companies smaller than IBM are stepping into the clinical trials arena. Wipro Technologies offers Wipro Clinical Collaboration Portal to drug developers, CROs, clinical sites, and regulators. The aim is to “significantly improve collaboration capabilities for multiregion clinical trials.”The Wipro platform was thus designed to reduce the clinical trial cycle time by 20–30%, the company reported. The portal speeds up communication and document exchanges among the sponsor organization, CRO staff, clinical site coordinators, and principal investigators.PharmaPros provides technology solutions for data and workflow management in clinical trials. Its Dataflow Manager™ uses cloud computing, enabling trial managers to make more rapid and better-informed decisions during a trial.“We now have data streaming in from many disparate sources: Interactive voice response systems, electronic data capture, ePRO, laboratory data, imaging data, safety signaling systems,” PharmaPros’ head of strategic development Brion Regan told GEN. “What we’re left with is a challenge to view clinical trial data holistically, in stream, and in time to make critical decisions about the conduct of the trial.”“Because the data is broken up into disparate technologies and sources, the ability to quickly see trends, identify safety issues, or simply report on trial progress has become extremely difficult.” As a result, he noted, many life science organizations have taken on the herculean task of integrating all this disparate data into one repository in an effort to gain visibility.This is not, in PharmaPros’ opinion, a realistic solution for in-stream trial management. It believes the focus should instead be on gaining visibility without moving data. “What we’re seeing is companies attempting to integrate data, only to discover it’s incomplete or not ready; then they try again. It’s become a burdensome cycle for statisticians. It’s also just extremely inefficient.“For driving decisions and operations, clinical data doesn’t actually need to be integrated,” Regan noted. “Cloud-based solutions can leverage the information that resides in these disparate systems and deliver an integrated view of the data.”Cloud-based computing has already become integrated into drug R&D at many levels. In spite of security and privacy concerns, the benefits of leveraging cloud computing in the clinical trial process have proven to be enticing to drug developers, CROs, clinical site managers, and others involved.The ease of information sharing as well as enhanced collaborative and analytical capabilities are very attractive given the international nature of clinical trials. These advantages will prompt many companies to adopt it and adapt to it.GEN | News Highlights:Firms Report Promising Data for Type 1 and 2 Diabetes Candidates at 71st Annual ADA Meeting
To contact us Click HERE
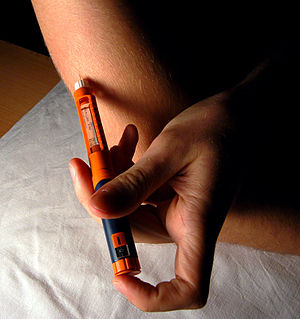 Image via WikipediaGEN | News Highlights:Firms Report Promising Data for Type 1 and 2 Diabetes Candidates at 71st Annual ADA Meeting
Image via WikipediaGEN | News Highlights:Firms Report Promising Data for Type 1 and 2 Diabetes Candidates at 71st Annual ADA Meeting

 Image via WikipediaGEN | News Highlights:Firms Report Promising Data for Type 1 and 2 Diabetes Candidates at 71st Annual ADA Meeting
Image via WikipediaGEN | News Highlights:Firms Report Promising Data for Type 1 and 2 Diabetes Candidates at 71st Annual ADA Meeting- The American Diabetes Association (ADA) meeting in San Diego included the presentation of new data from Phase III trials evaluating Bristol-Myers Squibb (BMS) and AstraZeneca’s dapagliflozin, Boehringer Ingelheim and Eli Lilly’s linagliptin, and Novo Nordisk’s insulin degludec. BMS and AstraZeneca reported positive long-term data from a Phase III clinical study evaluating their investigational sodium-glucose co-transporter-2 (SGLT2) inhibitor dapagliflozin, combined with metformin, for the treatment of type 2 diabetes. Data from the trial showed that the dapagliflozin-metformin combination maintained reductions in HbA1c levels from 52-weeks to 104-weeks, compared with combination therapy using the sulfonylurea glipizide and metformin. The study data also confirmed that patients treated with dapagliflozin added to metformin experienced 10 times less frequent hypoglycemic episodes than those treated using glipizide added to metformin. Participants in the dapagliflozin arm also maintained the weight loss they had achieved at 52 weeks through to 104 weeks, while patients in the glipizide therapy arm gained weight in the initial 52-week trial, and sustained this through to 104 weeks. The firms say the 104-week results are the longest-term clinical data presented to date for an SGLT2 inhibitor. A Marketing Authorization Application for dapagliflozin was validated by the European Medicines Agency in January. FDA accepted an NDA for the drug in March 2011, and has set the PDUFA date for October 28. BMS and AstraZeneca established their type 2 diabetes drug development collaboration back in January 2007. Boehringer Ingelheim and partner Eli Lilly, meanwhile, reported positive data from a Phase III study evaluating linagliptin (proposed trade name Trajenta® in Europe) combined with metformin in the treatment of type 2 diabetes. The results showed that the linagliptin combination therapy was as effective as glimepiride plus metformin in terms of lowering HbA1c levels over two years, but also led to a significantly lower incidence of hypoglycemia (7.5% vs. 36.1%, respectively) and a 54% relative risk reduction for cardiovascular events. Patients treated using linagliptin plus metformin also lost weight over two years, while those in the glimepiride-metformin treatment arm gained weight. Additional data from a pooled analysis of three Phase III studies showed that patients treated using linagliptin had HbA1c reductions independent of renal function, and in patients with severe renal impairment whose blood glucose levels weren’t otherwise sufficiently controlled, linagliptin provided clinically meaningful HbA1c reductions after 12 weeks of treatment. BI and Lilly established a collaboration to co-develop specific type 2 diabetes candidates in their respective pipelines, in January. Novo Nordisk’s presentations at the ADA meeting included data from two Phase III 52-week trials evaluating the ultra long-acting insulin, degludec, in patients with both type 1 and type 2 diabetes. The results showed that insulin degludec therapy led to HbA1c reductions of 0.4% in patients with type 1 diabetes, and 1.2% in those with type 2 diabetes, which was statistically noninferior to therapy with insulin glargine. In type 2 diabetes patients insulin degludec therapy was also associated with statistically fewer hypoglycemic events, averaging at 11.1 episodes per patient per year, compared with 13.6 episodes per patient per year for participants treated using insulin glargine. Rates of nocturnal hypoglycemia were 25% lower among both type 1 and type 2 diabetes patients receiving insulin degludec than among those taking insulin glargine. Additional trial data presented at the ADA meeting showed that insulin degludec could be dosed at different times from day to day (up to 40 hours apart) in type 2 diabetes patients without affecting overall glycemic control or risk of hypglycemia, compared with insulin glargine.
Related articles
- Bristol-Myers' pioneering diabetes drug bests rivals in 2-year study (fiercebiotech.com)
- ADA Conference 2011: The Good, The Bad, and the Errr... News (diabetesmine.com)
- Abilify and Seroquel makers AstraZeneca, Bristol-Myers Squibb diabetes drug dapagliflozin linked to breast and bladder cancer (bipolarsoupkitchen-stephany.blogspot.com)
- Life expectancy for those with Type 1 diabetes improving, study says (medicalxpress.com)

Pfizer's To Present Lung Cancer Data July 3-7
To contact us Click HERE
 Image via CrunchBase Pfizer Inc. will present early and mid-stage data from its lung cancer portfolio, including PF-00299804 (PF-299) an investigational, oral, pan-HER inhibitor;1 and crizotinib, an investigational, oral, first-in-class compound that inhibits the anaplastic lymphoma kinase, or ALK,2 at the International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), July 3-7 in Amsterdam, The Netherlands.
Image via CrunchBase Pfizer Inc. will present early and mid-stage data from its lung cancer portfolio, including PF-00299804 (PF-299) an investigational, oral, pan-HER inhibitor;1 and crizotinib, an investigational, oral, first-in-class compound that inhibits the anaplastic lymphoma kinase, or ALK,2 at the International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), July 3-7 in Amsterdam, The Netherlands.
“While lung cancer remains a difficult-to-treat disease, we’re learning more about how therapies like crizotinib and PF-299 may be able to specifically target ALK or the HER pathway, respectively, and how this may lead to more rationally selected and personalized therapy,” said Maurizio Voi, MD, Thoracic Tumor Strategy Lead, Pfizer Oncology. “Data being presented show survival outcomes for PF-299 and crizotinib, as well as quality-of-life or patient-reported outcomes after treatment for patients with non small cell lung cancer, which represent important considerations in determining the best treatment option for these patients.”
First Presentation of PF-299 Preliminary Overall Survival Data
Continued....
Pfizer will present, for the first time, preliminary overall survival data from a Phase 2 study evaluating PF-299 vs erlotinib in patients with advanced non-small cell lung cancer (NSCLC) after progression on at least one chemotherapy regimen (oral presentation, Abstract #745, Monday, July 4).1
Pfizer also will present patient-reported outcomes (PRO) from clinical trials of PF-299 in refractory and second-/third-line NSCLC, which provide a better understanding of the patient’s perspective of the burden of adverse events associated with treatment and how it may change over time.3,4
PF-299 targets multiple receptors of the HER pathway. PF-299 is an irreversible inhibitor of HER-1 (EGFR), HER-2 and HER-4 tyrosine kinases. 6
Crizotinib Data to be presented:
At the WCLC, data on the anti-tumor activity, safety, overall survival, patient-reported and quality-of-life outcomes observed in clinical trials of Pfizer’s crizotinib will be presented.2,7,8
About Pfizer Oncology
Pfizer Oncology is committed to the discovery, investigation and development of innovative treatment options to improve the outlook for cancer patients worldwide. Our strong pipeline, one of the most robust in the industry, is studied with precise focus on identifying and translating the best scientific breakthroughs into clinical application for patients across a wide range of cancers. Pfizer Oncology has biologics and small molecules in clinical development and more than 100 clinical trials underway. By working collaboratively with academic institutions, individual researchers, cooperative research groups, governments, and licensing partners, Pfizer Oncology strives to cure or control cancer with breakthrough medicines, to deliver the right drug for each patient at the right time. For more information please visit www.Pfizer.com.
DISCLOSURE NOTICE: The information contained in this release is as of June 28, 2011. Pfizer assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
This release contains forward-looking information about various oncology product candidates, including their potential benefits, that involves substantial risks and uncertainties. Such risks and uncertainties include, among other things, the uncertainties inherent in research and development; decisions by regulatory authorities regarding whether and when to approve any drug applications that have been or may be filed for any such oncology product candidates as well as their decisions regarding labeling and other matters that could affect their availability or commercial potential; and competitive developments.
A further description of risks and uncertainties can be found in Pfizer’s Annual Report on Form 10-K for the fiscal year ended December 31, 2010 and in its reports on Form 10-Q and Form 8-K.
1 World Lung Accepted Abstract #745. Overall Survival (OS) Results of a Randomized Phase 2 Trial of PF299804 versus Erlotinib in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC) After Failure of Chemotherapy. Oral Session, Monday July 4, 2011: 3:35 PM – 3:45 PM CEST. M. Boyer – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
2 World Lung Accepted Abstract #1618. Phase 2 Data for Crizotinib (PF-02341066) in ALK-Positive Advanced Non-Small Cell Lung Cancer (NSCLC): PROFILE 1005. Oral Session, Wednesday July 6, 2011: 3:10 PM – 3:20 PM CEST. G. Riely – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
3 World Lung Accepted Abstract #957. Gastrointestinal Toxicity of the Pan-HER Tyrosine Kinase Inhibitor (TKI) PF299804: Assessment by Patient-Reported Outcomes in 2nd/3rd-Line and Refractory Non-Small Cell Lung Cancer (NSCLC). Poster Session, Wednesday July 6, 2011: 12:15 PM – 2:15 PM CEST. A. Campbell – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
4 World Lung Accepted Abstract #702. Dermatologic Adverse Events of the Pan-HER Tyrosine Kinase Inhibitor (TKI) PF299804: Assessment by Patient-Reported Outcomes in 2nd/3rd-line and Refractory Non-Small Cell Lung Cancer (NSCLC). Poster Session, Wednesday July 6, 2011: 12:15 PM – 2:15 PM CEST. A. Campbell – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
5 Clinicaltrials.gov. ARCHER 1009: A Phase 3 Study of PF-00299804, a Pan-HER Inhibitor, Vs. Erolotinib in the Treatment of Advanced Non-Small Cell Lung Cancer. Available here: http://www.clinicaltrials.gov/ct2/show/NCT01360554?term=ARCHER&rank=1. Accessed June 21, 2011.
6 Gonzales AJ, Hook KE, Althaus IW et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbBreceptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7:1880-89.
7 World Lung Accepted Abstract #1510. PROFILE 1005: Preliminary Patient-Reported Outcomes (PROs) from an Ongoing Phase 2 Study of Crizotinib (PF-02341066) in Anaplastic Lymphoma Kinase (ALK)-Positive Advanced Non-Small Cell Lung Cancer (NSCLC). Oral Session, Wednesday July 6, 2011: 3:30 PM – 3:40 PM CEST. F. Blackhall – Presenter. Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
8 World Lung Accepted Abstract #1207. Crizotinib improves overall survival of ALK-positive patients with advanced NSCLC compared with historical controls. Oral Session, Wednesday July 6, 2011: 3:20 PM – 3:30 PM CEST. A. Shaw – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
9 World Lung Accepted Abstract #1349. Efficacy of Crizotinib in Retrospective Comparisons with Standard-Of-Care (SOC) Regimens from Three Pfizer-Sponsored Clinical Trials in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC). Poster Session, Wednesday July 6, 2011: 12:15 PM – 2:15 PM CEST. Y. Tang – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
10 Bang Y et al. Clinical Activity of the Oral ALK Inhibitor, Crizotinib (PF-02341066), in Patients with ALK-positive Non-Small Cell Lung Cancer. Accepted Plenary Presentation at the American Society of Clinical Oncology Annual Meeting, June 4-8, 2010. Chicago, IL.
11 Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-MET,
PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408-4417.
12 Chiarle R, Voena C, Ambrogio C et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1): 11-23.

 Image via CrunchBase Pfizer Inc. will present early and mid-stage data from its lung cancer portfolio, including PF-00299804 (PF-299) an investigational, oral, pan-HER inhibitor;1 and crizotinib, an investigational, oral, first-in-class compound that inhibits the anaplastic lymphoma kinase, or ALK,2 at the International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), July 3-7 in Amsterdam, The Netherlands.
Image via CrunchBase Pfizer Inc. will present early and mid-stage data from its lung cancer portfolio, including PF-00299804 (PF-299) an investigational, oral, pan-HER inhibitor;1 and crizotinib, an investigational, oral, first-in-class compound that inhibits the anaplastic lymphoma kinase, or ALK,2 at the International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), July 3-7 in Amsterdam, The Netherlands. “While lung cancer remains a difficult-to-treat disease, we’re learning more about how therapies like crizotinib and PF-299 may be able to specifically target ALK or the HER pathway, respectively, and how this may lead to more rationally selected and personalized therapy,” said Maurizio Voi, MD, Thoracic Tumor Strategy Lead, Pfizer Oncology. “Data being presented show survival outcomes for PF-299 and crizotinib, as well as quality-of-life or patient-reported outcomes after treatment for patients with non small cell lung cancer, which represent important considerations in determining the best treatment option for these patients.”
First Presentation of PF-299 Preliminary Overall Survival Data
Continued....
Pfizer will present, for the first time, preliminary overall survival data from a Phase 2 study evaluating PF-299 vs erlotinib in patients with advanced non-small cell lung cancer (NSCLC) after progression on at least one chemotherapy regimen (oral presentation, Abstract #745, Monday, July 4).1
Pfizer also will present patient-reported outcomes (PRO) from clinical trials of PF-299 in refractory and second-/third-line NSCLC, which provide a better understanding of the patient’s perspective of the burden of adverse events associated with treatment and how it may change over time.3,4
- Gastrointestinal toxicity of the pan-HER tyrosine kinase inhibitor (TKI) PF299804: Assessment by patient-reported outcomes in second-/third-line and refractory NSCLC (poster session, Abstract #957, Wednesday, July 6)3
- Dermatologic adverse events of the pan-HER tyrosine kinase inhibitor (TKI) PF299804: Assessment by patient-reported outcomes in second-/third-line and refractory NSCLC (poster session, Abstract #702, Wednesday, July 6)4
PF-299 targets multiple receptors of the HER pathway. PF-299 is an irreversible inhibitor of HER-1 (EGFR), HER-2 and HER-4 tyrosine kinases. 6
Crizotinib Data to be presented:
At the WCLC, data on the anti-tumor activity, safety, overall survival, patient-reported and quality-of-life outcomes observed in clinical trials of Pfizer’s crizotinib will be presented.2,7,8
- Phase 2 data for crizotinib in ALK-positive advanced NSCLC: PROFILE 1005 (oral presentation, Abstract #1618, Wednesday, July 6)2
- PROFILE 1005: Preliminary patient-reported outcomes (PROs) from an ongoing Phase 2 study of crizotinib in ALK-positive advanced NSCLC (oral presentation, Abstract #1510, Wednesday, July 6)7
- Crizotinib improves overall survival of ALK-positive patients with advanced NSCLC compared with historical controls (oral presentation, Abstract #1207, Wednesday, July 6)8
- Efficacy of crizotinib in retrospective comparisons with standard-of-care (SOC) regimens from three Pfizer-sponsored clinical trials in patients with advanced NSCLC (poster session, Abstract #1349, Wednesday, July 6)9
About Pfizer Oncology
Pfizer Oncology is committed to the discovery, investigation and development of innovative treatment options to improve the outlook for cancer patients worldwide. Our strong pipeline, one of the most robust in the industry, is studied with precise focus on identifying and translating the best scientific breakthroughs into clinical application for patients across a wide range of cancers. Pfizer Oncology has biologics and small molecules in clinical development and more than 100 clinical trials underway. By working collaboratively with academic institutions, individual researchers, cooperative research groups, governments, and licensing partners, Pfizer Oncology strives to cure or control cancer with breakthrough medicines, to deliver the right drug for each patient at the right time. For more information please visit www.Pfizer.com.
DISCLOSURE NOTICE: The information contained in this release is as of June 28, 2011. Pfizer assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
This release contains forward-looking information about various oncology product candidates, including their potential benefits, that involves substantial risks and uncertainties. Such risks and uncertainties include, among other things, the uncertainties inherent in research and development; decisions by regulatory authorities regarding whether and when to approve any drug applications that have been or may be filed for any such oncology product candidates as well as their decisions regarding labeling and other matters that could affect their availability or commercial potential; and competitive developments.
A further description of risks and uncertainties can be found in Pfizer’s Annual Report on Form 10-K for the fiscal year ended December 31, 2010 and in its reports on Form 10-Q and Form 8-K.
1 World Lung Accepted Abstract #745. Overall Survival (OS) Results of a Randomized Phase 2 Trial of PF299804 versus Erlotinib in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC) After Failure of Chemotherapy. Oral Session, Monday July 4, 2011: 3:35 PM – 3:45 PM CEST. M. Boyer – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
2 World Lung Accepted Abstract #1618. Phase 2 Data for Crizotinib (PF-02341066) in ALK-Positive Advanced Non-Small Cell Lung Cancer (NSCLC): PROFILE 1005. Oral Session, Wednesday July 6, 2011: 3:10 PM – 3:20 PM CEST. G. Riely – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
3 World Lung Accepted Abstract #957. Gastrointestinal Toxicity of the Pan-HER Tyrosine Kinase Inhibitor (TKI) PF299804: Assessment by Patient-Reported Outcomes in 2nd/3rd-Line and Refractory Non-Small Cell Lung Cancer (NSCLC). Poster Session, Wednesday July 6, 2011: 12:15 PM – 2:15 PM CEST. A. Campbell – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
4 World Lung Accepted Abstract #702. Dermatologic Adverse Events of the Pan-HER Tyrosine Kinase Inhibitor (TKI) PF299804: Assessment by Patient-Reported Outcomes in 2nd/3rd-line and Refractory Non-Small Cell Lung Cancer (NSCLC). Poster Session, Wednesday July 6, 2011: 12:15 PM – 2:15 PM CEST. A. Campbell – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
5 Clinicaltrials.gov. ARCHER 1009: A Phase 3 Study of PF-00299804, a Pan-HER Inhibitor, Vs. Erolotinib in the Treatment of Advanced Non-Small Cell Lung Cancer. Available here: http://www.clinicaltrials.gov/ct2/show/NCT01360554?term=ARCHER&rank=1. Accessed June 21, 2011.
6 Gonzales AJ, Hook KE, Althaus IW et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbBreceptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7:1880-89.
7 World Lung Accepted Abstract #1510. PROFILE 1005: Preliminary Patient-Reported Outcomes (PROs) from an Ongoing Phase 2 Study of Crizotinib (PF-02341066) in Anaplastic Lymphoma Kinase (ALK)-Positive Advanced Non-Small Cell Lung Cancer (NSCLC). Oral Session, Wednesday July 6, 2011: 3:30 PM – 3:40 PM CEST. F. Blackhall – Presenter. Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
8 World Lung Accepted Abstract #1207. Crizotinib improves overall survival of ALK-positive patients with advanced NSCLC compared with historical controls. Oral Session, Wednesday July 6, 2011: 3:20 PM – 3:30 PM CEST. A. Shaw – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
9 World Lung Accepted Abstract #1349. Efficacy of Crizotinib in Retrospective Comparisons with Standard-Of-Care (SOC) Regimens from Three Pfizer-Sponsored Clinical Trials in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC). Poster Session, Wednesday July 6, 2011: 12:15 PM – 2:15 PM CEST. Y. Tang – Presenter. International Association for the Study of Lung Cancer’s (IASLC) 14th World Conference on Lung Cancer (WCLC), Amsterdam, The Netherlands. July 3-7, 2011.
10 Bang Y et al. Clinical Activity of the Oral ALK Inhibitor, Crizotinib (PF-02341066), in Patients with ALK-positive Non-Small Cell Lung Cancer. Accepted Plenary Presentation at the American Society of Clinical Oncology Annual Meeting, June 4-8, 2010. Chicago, IL.
11 Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-MET,
PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408-4417.
12 Chiarle R, Voena C, Ambrogio C et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1): 11-23.
Related articles
- Pfizer Lung Cancer Drug to Get Priority Review (xconomy.com)
- Benefit of targeted lung cancer therapy confirmed (eurekalert.org)
- Pfizer drug shows double survival time for certain lung cancer patients (nj.com)
- Analyst bets on approval of Pfizer's lung cancer drug (fiercebiotech.com)

8 Temmuz 2012 Pazar
Anacor Pharmaceuticals and Psoriasis Make Strides With Phase IIb Trials
To contact us Click HERE
 Image via Wikipedia Anacor Pharmaceuticals (NASDAQ:ANAC) announced today preliminary results from its Phase 2b trial of AN2728 for the treatment of mild-to-moderate plaque-type psoriasis. The trial enrolled 68 subjects randomized in a 2:1 ratio, AN2728 to vehicle. Subjects treated with AN2728 showed improvement over vehicle at each of the recorded timepoints during the 12-week study period with peak efficacy of 26% occurring after six weeks of treatment with AN2728.
Image via Wikipedia Anacor Pharmaceuticals (NASDAQ:ANAC) announced today preliminary results from its Phase 2b trial of AN2728 for the treatment of mild-to-moderate plaque-type psoriasis. The trial enrolled 68 subjects randomized in a 2:1 ratio, AN2728 to vehicle. Subjects treated with AN2728 showed improvement over vehicle at each of the recorded timepoints during the 12-week study period with peak efficacy of 26% occurring after six weeks of treatment with AN2728.
This Phase 2b trial, while not powered to demonstrate statistical significance, was conducted under anticipated Phase 3 conditions to inform the design and conduct of fully-powered Phase 3 trials. The trial was designed to provide preliminary indications of efficacy and local tolerability and systemic safety when treating all or nearly all of the plaques on each subject. The previous four Phase 1 and three Phase 2 trials assessing the safety and efficacy of AN2728 had been designed to treat psoriasis on smaller body surface areas.
“AN2728 continues to show promise as a topical treatment for mild-to-moderate plaque-type psoriasis with the efficacy of a mid-potency steroid but without the side effects of a steroid or the tolerability issues of Vitamin D analogs,” said David Perry, Chief Executive Officer of Anacor Pharmaceuticals. “We saw improvement over vehicle throughout this Phase 2b study and observed no serious adverse events in the subjects treated with AN2728. The seven previous AN2728 trials demonstrated statistically significant efficacy and, combined with the results from this trial, provide us with the data to prepare us for our end of Phase 2 meeting with the FDA and to plan the Phase 3 trials of AN2728 for psoriasis.”
The trial was conducted at 10 sites in the United States on 68 subjects with a diagnosis of mild-to-moderate plaque-type psoriasis (defined as a Physicians’ Global Assessment (PGA) score of 2 or 3 out of 5), involving 2% to 35% of total body surface area. The trial design and endpoints were meant to simulate anticipated Phase 3 trial designs. Subjects were randomized to 2% AN2728 topical ointment or vehicle in a 2:1 ratio. Subjects applied the study medication to plaques twice a day for 12 weeks. Primary efficacy was defined as “Clear” (score of 0) or “Almost Clear” (score of 1) with at least a 2-grade improvement from baseline (“PGA Success”).
The highest proportion of subjects achieving PGA Success in the Intent to Treat analysis occurred at Day 42 with 26% of subjects in the AN2728 arm (n=46) achieving PGA Success compared to 18% in the vehicle arm (n=22). At the end of treatment (Day 84), 17% of the subjects in the AN2728 arm met PGA Success, while 14% of the vehicle group met that standard. The most common adverse events were “upper respiratory tract infection” (9.1% in the vehicle group), “contact dermatitis” (8.7% in the AN2728 group) and “nasopharyngitis” (8.7% in the AN2728 group and 4.5% in the vehicle group).
“These trial results demonstrate that AN2728 has the potential to make an important contribution to the treatment of psoriasis,” said Karl Beutner, M.D., Ph.D., and Chair of Anacor’s Clinical Advisory Board.
Psoriasis affects approximately 100 million people worldwide, with about 80% of cases being classified as “mild-to-moderate.” The majority of mild-to-moderate psoriasis patients are treated topically. Topical treatments for mild-to-moderate psoriasis consist primarily of corticosteroids, which have safety issues, and Vitamin D analogs, which can cause stinging and burning upon application. In 2009, sales of topical therapies for psoriasis were $1.1 billion in the seven major pharmaceutical markets. In 2010, 3.9 million prescriptions were written for topical therapies for psoriasis in the United States.
Conference Call and Webcast Information
Anacor will host a conference call at 8:00 a.m. EDT / 5:00 a.m. PDT on Tuesday, June 28, 2011 to discuss the results of the Phase 2b trial and development plans for AN2728. To access the conference call please dial (877) 291-1367 (domestic) or (914) 495-8534 (international) and provide the Conference ID 78602622 or you may listen to the webcast, live on our website under Investors at www.anacor.com.
About AN2728
AN2728 is a boron-based small-molecule compound that inhibits the activity of phosphodiesterase-4 (PDE4), thereby reducing the production of TNF-alpha, IL-12, IL-23 and other pro-inflammatory cytokines that are the precursors of the inflammation associated with psoriasis.
About Anacor Pharmaceuticals
Anacor is a biopharmaceutical company focused on discovering, developing and commercializing novel small-molecule therapeutics derived from its boron chemistry platform. Anacor has five compounds in clinical development, all of which were internally discovered, including its three lead programs: AN2690, a topical antifungal for the treatment of onychomycosis; AN2728, a topical anti-inflammatory PDE-4 inhibitor for the treatment of psoriasis and atopic dermatitis; and GSK 2251052, or GSK ‘052 (formerly referred to as AN3365), a systemic antibiotic for the treatment of infections caused by Gram-negative bacteria, which has been licensed to GlaxoSmithKline under the companies' research and development agreement. In addition, Anacor is developing AN2718 as a topical antifungal product candidate for the treatment of onychomycosis and skin fungal infections, and AN2898 as a topical anti-inflammatory product candidate for the treatment of psoriasis and atopic dermatitis.

 Image via Wikipedia Anacor Pharmaceuticals (NASDAQ:ANAC) announced today preliminary results from its Phase 2b trial of AN2728 for the treatment of mild-to-moderate plaque-type psoriasis. The trial enrolled 68 subjects randomized in a 2:1 ratio, AN2728 to vehicle. Subjects treated with AN2728 showed improvement over vehicle at each of the recorded timepoints during the 12-week study period with peak efficacy of 26% occurring after six weeks of treatment with AN2728.
Image via Wikipedia Anacor Pharmaceuticals (NASDAQ:ANAC) announced today preliminary results from its Phase 2b trial of AN2728 for the treatment of mild-to-moderate plaque-type psoriasis. The trial enrolled 68 subjects randomized in a 2:1 ratio, AN2728 to vehicle. Subjects treated with AN2728 showed improvement over vehicle at each of the recorded timepoints during the 12-week study period with peak efficacy of 26% occurring after six weeks of treatment with AN2728. This Phase 2b trial, while not powered to demonstrate statistical significance, was conducted under anticipated Phase 3 conditions to inform the design and conduct of fully-powered Phase 3 trials. The trial was designed to provide preliminary indications of efficacy and local tolerability and systemic safety when treating all or nearly all of the plaques on each subject. The previous four Phase 1 and three Phase 2 trials assessing the safety and efficacy of AN2728 had been designed to treat psoriasis on smaller body surface areas.
“AN2728 continues to show promise as a topical treatment for mild-to-moderate plaque-type psoriasis with the efficacy of a mid-potency steroid but without the side effects of a steroid or the tolerability issues of Vitamin D analogs,” said David Perry, Chief Executive Officer of Anacor Pharmaceuticals. “We saw improvement over vehicle throughout this Phase 2b study and observed no serious adverse events in the subjects treated with AN2728. The seven previous AN2728 trials demonstrated statistically significant efficacy and, combined with the results from this trial, provide us with the data to prepare us for our end of Phase 2 meeting with the FDA and to plan the Phase 3 trials of AN2728 for psoriasis.”
The trial was conducted at 10 sites in the United States on 68 subjects with a diagnosis of mild-to-moderate plaque-type psoriasis (defined as a Physicians’ Global Assessment (PGA) score of 2 or 3 out of 5), involving 2% to 35% of total body surface area. The trial design and endpoints were meant to simulate anticipated Phase 3 trial designs. Subjects were randomized to 2% AN2728 topical ointment or vehicle in a 2:1 ratio. Subjects applied the study medication to plaques twice a day for 12 weeks. Primary efficacy was defined as “Clear” (score of 0) or “Almost Clear” (score of 1) with at least a 2-grade improvement from baseline (“PGA Success”).
The highest proportion of subjects achieving PGA Success in the Intent to Treat analysis occurred at Day 42 with 26% of subjects in the AN2728 arm (n=46) achieving PGA Success compared to 18% in the vehicle arm (n=22). At the end of treatment (Day 84), 17% of the subjects in the AN2728 arm met PGA Success, while 14% of the vehicle group met that standard. The most common adverse events were “upper respiratory tract infection” (9.1% in the vehicle group), “contact dermatitis” (8.7% in the AN2728 group) and “nasopharyngitis” (8.7% in the AN2728 group and 4.5% in the vehicle group).
“These trial results demonstrate that AN2728 has the potential to make an important contribution to the treatment of psoriasis,” said Karl Beutner, M.D., Ph.D., and Chair of Anacor’s Clinical Advisory Board.
Psoriasis affects approximately 100 million people worldwide, with about 80% of cases being classified as “mild-to-moderate.” The majority of mild-to-moderate psoriasis patients are treated topically. Topical treatments for mild-to-moderate psoriasis consist primarily of corticosteroids, which have safety issues, and Vitamin D analogs, which can cause stinging and burning upon application. In 2009, sales of topical therapies for psoriasis were $1.1 billion in the seven major pharmaceutical markets. In 2010, 3.9 million prescriptions were written for topical therapies for psoriasis in the United States.
Conference Call and Webcast Information
Anacor will host a conference call at 8:00 a.m. EDT / 5:00 a.m. PDT on Tuesday, June 28, 2011 to discuss the results of the Phase 2b trial and development plans for AN2728. To access the conference call please dial (877) 291-1367 (domestic) or (914) 495-8534 (international) and provide the Conference ID 78602622 or you may listen to the webcast, live on our website under Investors at www.anacor.com.
About AN2728
AN2728 is a boron-based small-molecule compound that inhibits the activity of phosphodiesterase-4 (PDE4), thereby reducing the production of TNF-alpha, IL-12, IL-23 and other pro-inflammatory cytokines that are the precursors of the inflammation associated with psoriasis.
About Anacor Pharmaceuticals
Anacor is a biopharmaceutical company focused on discovering, developing and commercializing novel small-molecule therapeutics derived from its boron chemistry platform. Anacor has five compounds in clinical development, all of which were internally discovered, including its three lead programs: AN2690, a topical antifungal for the treatment of onychomycosis; AN2728, a topical anti-inflammatory PDE-4 inhibitor for the treatment of psoriasis and atopic dermatitis; and GSK 2251052, or GSK ‘052 (formerly referred to as AN3365), a systemic antibiotic for the treatment of infections caused by Gram-negative bacteria, which has been licensed to GlaxoSmithKline under the companies' research and development agreement. In addition, Anacor is developing AN2718 as a topical antifungal product candidate for the treatment of onychomycosis and skin fungal infections, and AN2898 as a topical anti-inflammatory product candidate for the treatment of psoriasis and atopic dermatitis.
Related articles
- Anacor Pharmaceuticals Announces The Initiation Of A Phase 2 Trial Of AN2728 And AN2898 In Mild-To-Moderate Atopic Dermatitis (medicalnewstoday.com)
- What is psoriasis? (communityacupuncturist.wordpress.com)
- Psoriasis Cream (myjournal4all.wordpress.com)

Killer E. coli Strain Identified in German Outbreak Through Genomic Sequencing: Life Technologies
To contact us Click HERE
 Image via WikipediaAs of June 15, the number of people who were ill due to the outbreak of a new E. coli strain in Europe had reached 3,244, according to the Associated Press (AP). Most of the reports have been in Germany, and 784 of the total had developed a serious complication that could lead to kidney failure. AP reported that 37 people have died in Germany, and one person died in Sweden.
Image via WikipediaAs of June 15, the number of people who were ill due to the outbreak of a new E. coli strain in Europe had reached 3,244, according to the Associated Press (AP). Most of the reports have been in Germany, and 784 of the total had developed a serious complication that could lead to kidney failure. AP reported that 37 people have died in Germany, and one person died in Sweden.
The German government has said that it could be a while before the outbreak could be tracked to its ultimate source. Critics have lost no time carping about the way the crisis has been handled.
While the epidemiology has been cumbersome, the genomic sequencing that characterized the pathogen wasn’t. It took all of three days for teams at the University Medical Centre Hamburg-Eppendorf and BGI (formerly known as Beijing Institute of Genomics) to complete the sequence. The research groups used Life Technologies’ Ion Personal Genome Machine (PGM). Simultaneously, University Hospital of Muenster in Germany and Life Technologies confirmed these results.
 Image via Wikipedia
Image via Wikipedia
Described as super-toxic, the strain has 93% sequence similarity with the enteroaggregative E. coli (EAEC) 55989 strain isolated in the Central African Republic and known for causing serious diarrhea. Furthermore, the scientists reported, it has “acquired specific sequences that appear to be similar to those involved in the pathogenicity of hemorrhagic colitis and hemolytic-uremic syndrome.”
 Image via Wikipedia
Image via Wikipedia
The bacteria also produce extended-spectrum beta-lactamases, enzymes that confer resistance to most beta-lactam antibiotics including penicillins, cephalosporins, and the monobactam aztreonam.
University Hospital of Muenster and Life Technologies further noted that “the bacterium at the root of the deadly outbreak in Germany is a new hybrid type of pathogenic E. coli strain.” Their data reportedly showed the presence of genes typically found in two different types of E. coli: EAEC and EHEC.
A high-density array of wells on the ion semiconductor chips provides millions of individual reactors, while integrated fluidics allows reagents to flow over the sensor array. The combination, Life Technologies says, enables the direct translation of genetic information to digital information.
 Image via Wikipedia
Image via Wikipedia
“What’s different about it is that it doesn’t use cameras or lasers,” Life Technologies CEO Mark Stevenson told GEN. “We measure the pH change in each well with a semiconductor chip.”
The E. coli crisis in Germany appears to have provided the proof of principle and a field test with highly visible results for the Ion PGM. Life Technologies acquired the technology when it bought Ion Torrent in 2010 for $375 million in cash and stock. Life Technologies began selling the instrument to the marketplace for less than $100,000, calling it an “easy-to-use, highly accurate benchtop instrument optimal for mid-scale sequencing projects such as targeted and microbial sequencing.”
When Ion Torrent introduced the technology in 2007, it expected the innovation to democratize research, enabling every lab to have a sequencer on its bench. At the time, the company said it had planned to sell the machine for $50,000 apiece—about one-tenth the cost of existing instruments. Ion said that the instrument could perform a single sequencing “run” for about $500 in one hour.
 Image via Wikipedia
Image via Wikipedia
On June 6, shortly after reporting results from its sequencing efforts, the firm reported that it had completed development of a custom assay to detect the bacterium. Shipments of the TaqMan® E. coli O104 detection kit are now in Europe.
“A qPCR-based assay test is the most accurate method to detect harmful food-borne pathogens because a positive result indicates the presence of that particular strain’s DNA in the food sample that is being tested,” explained Nir Nimrodi, head of food safety at Life Technologies.
 Image via Wikipedia
Image via Wikipedia
“It is also the fastest. While traditional laboratory testing methods can take up to 10 days for results, this test can determine the presence or absence of the European pathogen in 10 to 24 hours, depending on the sample type and size.”
It is hoped that the TaqMan E. coli O104 detection kits will provide an answer to the question that has received a variety of answers since the initial E. coli outbreak: Where did it come from? Originally, on May 26, health officials pointed to E. coli-contaminated cucumbers from Spain as culprits, but researchers later concluded that the cucumbers were contaminated with a different strain.
Suspicion then moved to bean sprouts but faded away after it was found that 23 of 40 samples from the suspect farm tested negative. As of June 8, focus had shifted back to cucumbers, as a CUCO (cucumber of unknown country origin) that had sickened a family in eastern Germany was found to be contaminated with the outbreak strain. The cucumber was discovered in the family’s compost, but there is no conclusive evidence that it’s the source.
Then on June 10, Reinhard Burger, president of the Robert Koch Institute, said that even though no tests of the sprouts from the suspect farm had come back positive for the E. coli strain behind the outbreak, an investigation into the pattern of the outbreak had produced enough evidence to indict the sprouts. German authorities said that they haven’t yet been able to resolve how sprouts at a farm became contaminated.
Along the search for clues about the source of the killer E. coli food bug, a restaurant in the northern German town of Luebeck and a festival in the northern city of Hamburg have also come under suspicion. The European Union has sent food safety experts to Germany to help authorities there identify the source of the deadly E. coli epidemic.
Stevenson noted that “a concern for the global healthcare industry is the ability to identify these pathogens as they arise and then be able to detect and screen for them rapidly and inexpensively.” Like with the Ion PGM, as novel pathogens continue to emerge, so will the need for similar disruptive but relatively affordable technologies.
 Image via Wikipedia
Image via Wikipedia
 Image via Wikipedia
Image via Wikipedia

 Image via WikipediaAs of June 15, the number of people who were ill due to the outbreak of a new E. coli strain in Europe had reached 3,244, according to the Associated Press (AP). Most of the reports have been in Germany, and 784 of the total had developed a serious complication that could lead to kidney failure. AP reported that 37 people have died in Germany, and one person died in Sweden.
Image via WikipediaAs of June 15, the number of people who were ill due to the outbreak of a new E. coli strain in Europe had reached 3,244, according to the Associated Press (AP). Most of the reports have been in Germany, and 784 of the total had developed a serious complication that could lead to kidney failure. AP reported that 37 people have died in Germany, and one person died in Sweden. The German government has said that it could be a while before the outbreak could be tracked to its ultimate source. Critics have lost no time carping about the way the crisis has been handled.
While the epidemiology has been cumbersome, the genomic sequencing that characterized the pathogen wasn’t. It took all of three days for teams at the University Medical Centre Hamburg-Eppendorf and BGI (formerly known as Beijing Institute of Genomics) to complete the sequence. The research groups used Life Technologies’ Ion Personal Genome Machine (PGM). Simultaneously, University Hospital of Muenster in Germany and Life Technologies confirmed these results.
 Image via Wikipedia
Image via WikipediaInformation Uncovered by Sequencing
“Bioinformatics analysis revealed that this E. coli is a new strain of bacteria that is highly infectious and toxic,” BGI said in its report, issued on June 2. The bacterium is an enterohemorrhagic E. coli (EHEC) serotype O104 strain, which has never been involved in any E. coli outbreaks before, the institute explained.Described as super-toxic, the strain has 93% sequence similarity with the enteroaggregative E. coli (EAEC) 55989 strain isolated in the Central African Republic and known for causing serious diarrhea. Furthermore, the scientists reported, it has “acquired specific sequences that appear to be similar to those involved in the pathogenicity of hemorrhagic colitis and hemolytic-uremic syndrome.”
 Image via Wikipedia
Image via WikipediaThe bacteria also produce extended-spectrum beta-lactamases, enzymes that confer resistance to most beta-lactam antibiotics including penicillins, cephalosporins, and the monobactam aztreonam.
University Hospital of Muenster and Life Technologies further noted that “the bacterium at the root of the deadly outbreak in Germany is a new hybrid type of pathogenic E. coli strain.” Their data reportedly showed the presence of genes typically found in two different types of E. coli: EAEC and EHEC.
The Instrument that Did the Sequencing
The Ion PGM chip-based sequencing technology connects chemical and digital information through the use of semiconductor technology. It uses a parallel array of semiconductor sensors to perform real-time measurement of the hydrogen ions produced during DNA replication.A high-density array of wells on the ion semiconductor chips provides millions of individual reactors, while integrated fluidics allows reagents to flow over the sensor array. The combination, Life Technologies says, enables the direct translation of genetic information to digital information.
 Image via Wikipedia
Image via Wikipedia“What’s different about it is that it doesn’t use cameras or lasers,” Life Technologies CEO Mark Stevenson told GEN. “We measure the pH change in each well with a semiconductor chip.”
The E. coli crisis in Germany appears to have provided the proof of principle and a field test with highly visible results for the Ion PGM. Life Technologies acquired the technology when it bought Ion Torrent in 2010 for $375 million in cash and stock. Life Technologies began selling the instrument to the marketplace for less than $100,000, calling it an “easy-to-use, highly accurate benchtop instrument optimal for mid-scale sequencing projects such as targeted and microbial sequencing.”
When Ion Torrent introduced the technology in 2007, it expected the innovation to democratize research, enabling every lab to have a sequencer on its bench. At the time, the company said it had planned to sell the machine for $50,000 apiece—about one-tenth the cost of existing instruments. Ion said that the instrument could perform a single sequencing “run” for about $500 in one hour.
 Image via Wikipedia
Image via WikipediaDetection in Food
The availability of new technologies has indeed greatly facilitated the genomic sequencing of this potentially lethal bacterium. Life Technologies has also developed a tool to test foods thought to be associated with the outbreak.On June 6, shortly after reporting results from its sequencing efforts, the firm reported that it had completed development of a custom assay to detect the bacterium. Shipments of the TaqMan® E. coli O104 detection kit are now in Europe.
“A qPCR-based assay test is the most accurate method to detect harmful food-borne pathogens because a positive result indicates the presence of that particular strain’s DNA in the food sample that is being tested,” explained Nir Nimrodi, head of food safety at Life Technologies.
 Image via Wikipedia
Image via Wikipedia“It is also the fastest. While traditional laboratory testing methods can take up to 10 days for results, this test can determine the presence or absence of the European pathogen in 10 to 24 hours, depending on the sample type and size.”
It is hoped that the TaqMan E. coli O104 detection kits will provide an answer to the question that has received a variety of answers since the initial E. coli outbreak: Where did it come from? Originally, on May 26, health officials pointed to E. coli-contaminated cucumbers from Spain as culprits, but researchers later concluded that the cucumbers were contaminated with a different strain.
Suspicion then moved to bean sprouts but faded away after it was found that 23 of 40 samples from the suspect farm tested negative. As of June 8, focus had shifted back to cucumbers, as a CUCO (cucumber of unknown country origin) that had sickened a family in eastern Germany was found to be contaminated with the outbreak strain. The cucumber was discovered in the family’s compost, but there is no conclusive evidence that it’s the source.
Then on June 10, Reinhard Burger, president of the Robert Koch Institute, said that even though no tests of the sprouts from the suspect farm had come back positive for the E. coli strain behind the outbreak, an investigation into the pattern of the outbreak had produced enough evidence to indict the sprouts. German authorities said that they haven’t yet been able to resolve how sprouts at a farm became contaminated.
Along the search for clues about the source of the killer E. coli food bug, a restaurant in the northern German town of Luebeck and a festival in the northern city of Hamburg have also come under suspicion. The European Union has sent food safety experts to Germany to help authorities there identify the source of the deadly E. coli epidemic.
Stevenson noted that “a concern for the global healthcare industry is the ability to identify these pathogens as they arise and then be able to detect and screen for them rapidly and inexpensively.” Like with the Ion PGM, as novel pathogens continue to emerge, so will the need for similar disruptive but relatively affordable technologies.
 Image via Wikipedia
Image via Wikipedia  Image via Wikipedia
Image via WikipediaRelated articles
- Sequencing Killer E. Coli Reveals New Strain (technologyreview.com)
- New Technology Helps Fight Deadly Outbreaks (usnews.com)
- DNA Sequence Yields Clues to Germany's 'Super Toxic' E. coli outbreak (news.sciencemag.org)
- How Genetic Engineering May Have Created E. Coli Outbreak. (ascleses.wordpress.com)
- Complete map of Germany E. coli O104 genome released (news.bioscholar.com)
- Europe's E. Coli Outbreak Caused By Mutant Bacteria Never Before Seen (healthland.time.com)
- Life Technologies' Ion Machine Speeds Analyses of Deadly European Illness (xconomy.com)

Cancer Clinical Trials and Treatments Late Stage Developments
To contact us Click HERE
 Image via WikipediaFrom FierceBiotech
Image via WikipediaFrom FierceBiotech
For the cancer drug research enthusiast, this report might read in places like a special oncology edition of a gun magazine. Indeed, there are plenty of weapons against cancer to read about here. Several of the drugs listed here represent the advancement of relatively new methods of attacking cancer, including "armed antibodies" and cancer-killing viruses.
In addition, decades of basic research into the molecular drivers of cancer growth are bearing fruit for drug developers. Not only are these companies making progress in clinical trials, they have landed buyout deals and lucrative partnerships. It's also clear that relatively small companies like Aveo Pharmaceuticals and Curis are making inroads along side the big boys like Pfizer and Roche.
There are 10 late-stage drugs listed in this report, but this editor hesitates to call them the "Top 10" only because there are so many variables to consider to rank them in such a way objectively. Yet these 10 drugs have certainly been generating news and, in most cases, lots of interest among investors and the medical community. All of the drugs have reached pivotal trials for at least one type of cancer.
Here's the list in alphabetical order by each drug's most commonly used moniker, whether that is its alphanumeric code name or generic name. As always, please let us know whether you think there are cancer drugs in pivotal trials that you think should have been on this list or ones on this list that shouldn't be.
1. Carfilzomib - multiple myeloma
2. Crizotinib (PF-02341066) - lung cancer
3. GDC-0449 (vismodegib) - basal cell carcinoma
4. OncoVex - advanced melanoma
5. PLX4032 (RG7204) - melanoma
6. Ponatinib - leukemia
7. SGN-35 (brentuximab vedotin) - Hodgkin's lymphoma, anaplastic large cell lymphomas
8. Tivozanib (AV-951) - advanced renal cell carcinoma
9. T-DM1 (Trastuzumab-DM1) - breast cancer
10. XL184 (cabozantinib) - prostate cancer
Onyx (ONXX)
 Image via CrunchBase
Image via CrunchBase
While Onyx Pharmaceuticals ($ONXX) has an approved kidney and liver cancer drug in Nexavar (sorafenib), much of the company's future value growth seems to be tied to its late-stage proteasome inhibitor carfilzomib.
In multiple myeloma, carfilzomib could offer some advantages over existing treatments for the plasma cancer such as reduced nerve damage, according to Onyx. The drug works by selectively inhibiting a protein complex in cells called the proteasome in order to make cancer cells more vulnerable to cell death. In December the firm revealed some promising Phase II data, showing that 24 percent of patients who took the drug after their cancers failed to respond to other therapies had at least a partial response.
Onyx is now conducting Phase III trials that aim to support its bids for approval of the drug for multiple myeloma in the U.S. and Europe. The company said it expanded its European trial in March, bringing the number of patients in the study from 84 to 300 and making overall survival the primary goal of the study rather than progression-free survival-a move that analysts at J.P. Morgan Research viewed as a positive because demonstrating survival benefits could differentiate the drug label among those of other myeloma treatments.
Onyx expects to complete submission of its application for FDA approval of the drug by mid-2011.
Pfizer (PFE) Image via CrunchBaseThe knock on Pfizer's ($PFE) crizotinib is that it only targets a tiny percentage of lung cancer patients, but the important thing is that the drug appears to have an impact for that tiny percentage.
Image via CrunchBaseThe knock on Pfizer's ($PFE) crizotinib is that it only targets a tiny percentage of lung cancer patients, but the important thing is that the drug appears to have an impact for that tiny percentage.
Pfizer seems to be on schedule to complete a submission in the first half of this year for FDA approval of the drug for patients whose non-small cell lung cancer expressed the anaplastic lymphoma kinase (ALK) gene, which is found in 3-5 percent of patients with the disease. While potential sales of this drug aren't expected to reach blockbuster levels, Pfizer has signaled through its emphasis on targeted drug development and investments in rare diseases treatments that it's happy to be in niche markets with important new drugs.
Last year Pfizer provided details from an early-state clinical data showing that 46 of 82 patients with NSCLC who took the drug had a partial response to the drug and one patient had a complete response. The company also reported last year that patients on averaged lived for 9.2 months without their cancer getting worse. A key in these studies was making sure that patients who took the drug had cancer with ALK mutations. When present, the mutations drive tumor growth and survival.
Curis (CRIS) Image via CrunchBase GDC-0449 might be one of many cancer drugs in the pipeline at Genentech, but for its partner Lexington, MA-based Curis ($CRIS) this drug is the most advanced compound in its pipeline. Swiss drug giant Roche, the owner of Genentech, has told Curis that it could make at least one regulatory submission for approval for the compound as a treatment for a common form of skin cancer called basal cell carcinoma (BCC) in 2011.
Image via CrunchBase GDC-0449 might be one of many cancer drugs in the pipeline at Genentech, but for its partner Lexington, MA-based Curis ($CRIS) this drug is the most advanced compound in its pipeline. Swiss drug giant Roche, the owner of Genentech, has told Curis that it could make at least one regulatory submission for approval for the compound as a treatment for a common form of skin cancer called basal cell carcinoma (BCC) in 2011.
In March, Curis announced that Genentech's Phase II study of the drug in inoperable BCC patients met its primary goal of shrinking tumors of a certain percentage of patients. This was good news after the drug flunked in mid-stage studies Genentech conducted in patients with ovarian and colorectal cancers last year. Curis, which does not yet have a drug on the market, is eligible for up to $115 million in milestone payments through this program with Roche/Genentech as well as single-digit royalties on potential sales.
Amgen (AMGN)
Amgen ($AMGN) made a huge bet on OncoVex in January with the buyout of the drug's Woburn, MA-based developer, BioVex, in a deal worth up to $1 billion. Later this year Amgen expects to see primary outcome data from a Phase III trial of the drug in patients with advanced melanoma, according to ClinicalTrials.gov. The company can only hope that the trial yields results as impressive as those from a small mid-stage trial in patients with the deadly skin cancer.
OncoVex attacks tumors in two ways. It features a modified cold sore virus that replicates inside solid tumors, causing cancer cells to die. Secondly, the drug prompts the immune system to take out cancer cells. In May 2009, BioVex impressed with Phase II data on the drug in melanoma patients, showing that of 13 patients who had significant responses to the treatment, nine had signs of the cancer completely wiped out.
Amgen is eying multiple markets for OncoVex, a late-stage trial for which is now recruiting patients with head and neck cancers. The drug has also shown potential to treat patients with breast and pancreatic cancers.
Daiichi Sankyo (DSKYF)
 Image via Wikipedia
Image via Wikipedia
Plexxikon is sitting pretty right now, largely due to the stellar performance of its lead experimental drug, code named PLX4032. After the Berkeley, CA-based firm and its partner Roche reported exciting data from trials of the drug in patients with melanoma over the past year, Japan's Daiichi Sankyo swooped in and bought Plexxikon in a $935 million deal revealed in February. What's more, Plexxikon CEO Peter Hirth told FierceBiotech that the company's Japanese owner plans to give the small drug developer a degree of autonomy and funding to continue its impressive work.
Plexxikon reported in January that melanoma patients on the drug in a Phase III trial were showing better overall survival and on average were living longer without their cancer progressing than those who were on the standard therapy for melanoma, dacarbazine. The drug is targeted for patients with a BFAF mutation. Roche is developing a DNA-based test to identify patients with the BRAF mutation, which causes unchecked cell growth and cancer. In general, BRAF mutations show up in most melanomas.
Plexxikon, a 2010 Fierce 15 company, published Phase I data in an August 2010 issue of the New England Journal of Medicine. The article said that 81 percent of melanoma patients on the drug had their tumors shrink by at least 30 percent.
Ariad Pharmaceuticals (ARIA)
 Image via Wikipedia
Image via Wikipedia
The big story at Cambridge, MA-based Ariad Pharmaceuticals ($ARIA) is its leukemia drug ponatinib, despite the fact that its lead experimental cancer drug is ridaforolimus (which is advancing in partnership with Merck). Ponatinib is now in a pivotal Phase II clinical trial as a treatment for patients with chronic myeloid leukemia or Philadelphia positive acute lymphoblastic leukemia whose cancers are resistant to Bristol-Myers Squibb's Sprycel or Novartis's Tasigna.
Ponatinib is designed to target multiple mutant forms of BCR-ABL fusion proteins, making it a potentially potent next-generation inhibitor of tyrosine kinases. In December the company reported that in a Phase I trial 66 percent of patients on the drug showed a major cytogenetic response, evidence that the drug was weakening their cancer (and such responses were shown in 100 percent of patients with a T315I mutation). The firm is interested in partnering on the development of ponatinib in certain markets outside of the U.S; so another pharma deal might be in the offing for the company.
Seattle Genetics (SGEN) Takeda Pharmaceuticals (TKPHF)
After promising results in clinical trials revealed late last year, SGN-35 (brentuximab vedotin) has a shot at being approved later this year for immune system cancers known as Hodgkin's lymphoma and anaplastic large cell lymphomas that have been resistant to prior therapies or come back after a period of time.
Seattle Genetics ($SGEN) and Millennium: The Takeda Oncology Company reported in December that the drug--which is an anti-CD30 monoclonal antibody linked to cancer-killing agent called an auristatin--completely wiped out visible signs of disease in just more than half of the 58 patients with forms of anaplastic large cell lymphomas in a Phase II study, with an additional third of the patients' tumors shrinking by at least half.
In a pivotal trial involving 102 patients with relapsed or resistant Hodgkin's lymphoma, the drug rendered the cancer undetectable in 34 percent of patients and provided partial remissions for 40 percent of volunteers in the trial. Seattle Genetics has U.S. and Canadian marketing rights to the drug, and Takeda controls commercialization of the treatment in the rest of the world.
Aveo Pharmaceuticals (AVEO) Image via CrunchBase
Image via CrunchBase
Tuan Ha-Ngoc, the president and CEO of Aveo Pharmaceuticals ($AVEO), is leading a very smart charge to advance tivozanib to the market for kidney cancers called advanced renal cell carcinoma (RCC).
In June 2010, the company showed results from a Phase II study of a the drug--a potent blocker of all three vascular endothelial growth factor (VEGF) receptors, which play a role in the formation of blood vessels that feed tumors--that patients taking the drug on average lived without their cancer getting worse for 11.8 months. That same key indicator of effectiveness was nearly 14.8 months among patients with a clear cell form of RCC who had prior surgeries to remove at least part of their kidneys.
In the company's 517-patient Phase III trial comparing tivozanib head-to-head with Onyx Pharmaceuticals' and Bayer's Nexavar, patients who enter the study must have a clear cell form of the disease and previously have had the surgery to be eligible (the types of patients who did really well in the Phase II study). Aveo plans to report results of the trial in mid-2011. It's also worth keeping an eye on Pfizer's similar drug, axitinib, for which the drug giant plans to submit in 2011 applications for U.S. and European regulatory approvals as a treatment for patients with kidney cancer.
Aveo is sharing equally North American and European development costs and profits related to tivozanib with Japan's Astellas Pharma. Kyowa Hakko Kirin has rights to develop and commercialize the drug in Asia.
ImmunoGen (IMGN)
Here's more evidence that linking targeting antibodies with cancer-killing drugs can pack a powerful one-two punch against tumors. This "armed antibody" drug due to be submitted (again) for regulatory approval in mid-2012. T-DM1 has been shining in clinical trials involving women with breast cancer for years, but the FDA handed back Swiss drug giant Roche's application for approval of the drug in August 2010. That application was made with promising Phase II data on the treatment, but regulators apparently want to see results from larger studies underway.
\T-DM1 is truly a two-pronged weapon against breast tumors. It uses linker technology from Waltham, MA-based ImmunoGen ($IMGN) to equip Roche's Herceptin, an antibody that binds to HER2-expressing breast tumors, with a cell-killing agent known as DM1. This enables the antibody to home in on the cancer target and deliver the cell-killing agent into tumor cells.
Preliminary data released in October from a 137-patient Phase II study showed that 47.8 percent of breast cancer patients on T-DM1 had a confirmed objective response on T-DM1, compared with 41.4 percent who were taking a combination of Herceptin and a chemotherapy drug, taxane.
Related articles

 Image via WikipediaFrom FierceBiotech
Image via WikipediaFrom FierceBiotech For the cancer drug research enthusiast, this report might read in places like a special oncology edition of a gun magazine. Indeed, there are plenty of weapons against cancer to read about here. Several of the drugs listed here represent the advancement of relatively new methods of attacking cancer, including "armed antibodies" and cancer-killing viruses.
In addition, decades of basic research into the molecular drivers of cancer growth are bearing fruit for drug developers. Not only are these companies making progress in clinical trials, they have landed buyout deals and lucrative partnerships. It's also clear that relatively small companies like Aveo Pharmaceuticals and Curis are making inroads along side the big boys like Pfizer and Roche.
There are 10 late-stage drugs listed in this report, but this editor hesitates to call them the "Top 10" only because there are so many variables to consider to rank them in such a way objectively. Yet these 10 drugs have certainly been generating news and, in most cases, lots of interest among investors and the medical community. All of the drugs have reached pivotal trials for at least one type of cancer.
Here's the list in alphabetical order by each drug's most commonly used moniker, whether that is its alphanumeric code name or generic name. As always, please let us know whether you think there are cancer drugs in pivotal trials that you think should have been on this list or ones on this list that shouldn't be.
1. Carfilzomib - multiple myeloma
2. Crizotinib (PF-02341066) - lung cancer
3. GDC-0449 (vismodegib) - basal cell carcinoma
4. OncoVex - advanced melanoma
5. PLX4032 (RG7204) - melanoma
6. Ponatinib - leukemia
7. SGN-35 (brentuximab vedotin) - Hodgkin's lymphoma, anaplastic large cell lymphomas
8. Tivozanib (AV-951) - advanced renal cell carcinoma
9. T-DM1 (Trastuzumab-DM1) - breast cancer
10. XL184 (cabozantinib) - prostate cancer
Onyx (ONXX)
 Image via CrunchBase
Image via CrunchBaseWhile Onyx Pharmaceuticals ($ONXX) has an approved kidney and liver cancer drug in Nexavar (sorafenib), much of the company's future value growth seems to be tied to its late-stage proteasome inhibitor carfilzomib.
In multiple myeloma, carfilzomib could offer some advantages over existing treatments for the plasma cancer such as reduced nerve damage, according to Onyx. The drug works by selectively inhibiting a protein complex in cells called the proteasome in order to make cancer cells more vulnerable to cell death. In December the firm revealed some promising Phase II data, showing that 24 percent of patients who took the drug after their cancers failed to respond to other therapies had at least a partial response.
Onyx is now conducting Phase III trials that aim to support its bids for approval of the drug for multiple myeloma in the U.S. and Europe. The company said it expanded its European trial in March, bringing the number of patients in the study from 84 to 300 and making overall survival the primary goal of the study rather than progression-free survival-a move that analysts at J.P. Morgan Research viewed as a positive because demonstrating survival benefits could differentiate the drug label among those of other myeloma treatments.
Onyx expects to complete submission of its application for FDA approval of the drug by mid-2011.
Pfizer (PFE)
 Image via CrunchBaseThe knock on Pfizer's ($PFE) crizotinib is that it only targets a tiny percentage of lung cancer patients, but the important thing is that the drug appears to have an impact for that tiny percentage.
Image via CrunchBaseThe knock on Pfizer's ($PFE) crizotinib is that it only targets a tiny percentage of lung cancer patients, but the important thing is that the drug appears to have an impact for that tiny percentage.Pfizer seems to be on schedule to complete a submission in the first half of this year for FDA approval of the drug for patients whose non-small cell lung cancer expressed the anaplastic lymphoma kinase (ALK) gene, which is found in 3-5 percent of patients with the disease. While potential sales of this drug aren't expected to reach blockbuster levels, Pfizer has signaled through its emphasis on targeted drug development and investments in rare diseases treatments that it's happy to be in niche markets with important new drugs.
Last year Pfizer provided details from an early-state clinical data showing that 46 of 82 patients with NSCLC who took the drug had a partial response to the drug and one patient had a complete response. The company also reported last year that patients on averaged lived for 9.2 months without their cancer getting worse. A key in these studies was making sure that patients who took the drug had cancer with ALK mutations. When present, the mutations drive tumor growth and survival.
Curis (CRIS)
 Image via CrunchBase GDC-0449 might be one of many cancer drugs in the pipeline at Genentech, but for its partner Lexington, MA-based Curis ($CRIS) this drug is the most advanced compound in its pipeline. Swiss drug giant Roche, the owner of Genentech, has told Curis that it could make at least one regulatory submission for approval for the compound as a treatment for a common form of skin cancer called basal cell carcinoma (BCC) in 2011.
Image via CrunchBase GDC-0449 might be one of many cancer drugs in the pipeline at Genentech, but for its partner Lexington, MA-based Curis ($CRIS) this drug is the most advanced compound in its pipeline. Swiss drug giant Roche, the owner of Genentech, has told Curis that it could make at least one regulatory submission for approval for the compound as a treatment for a common form of skin cancer called basal cell carcinoma (BCC) in 2011.In March, Curis announced that Genentech's Phase II study of the drug in inoperable BCC patients met its primary goal of shrinking tumors of a certain percentage of patients. This was good news after the drug flunked in mid-stage studies Genentech conducted in patients with ovarian and colorectal cancers last year. Curis, which does not yet have a drug on the market, is eligible for up to $115 million in milestone payments through this program with Roche/Genentech as well as single-digit royalties on potential sales.
Amgen (AMGN)
Amgen ($AMGN) made a huge bet on OncoVex in January with the buyout of the drug's Woburn, MA-based developer, BioVex, in a deal worth up to $1 billion. Later this year Amgen expects to see primary outcome data from a Phase III trial of the drug in patients with advanced melanoma, according to ClinicalTrials.gov. The company can only hope that the trial yields results as impressive as those from a small mid-stage trial in patients with the deadly skin cancer.
OncoVex attacks tumors in two ways. It features a modified cold sore virus that replicates inside solid tumors, causing cancer cells to die. Secondly, the drug prompts the immune system to take out cancer cells. In May 2009, BioVex impressed with Phase II data on the drug in melanoma patients, showing that of 13 patients who had significant responses to the treatment, nine had signs of the cancer completely wiped out.
Amgen is eying multiple markets for OncoVex, a late-stage trial for which is now recruiting patients with head and neck cancers. The drug has also shown potential to treat patients with breast and pancreatic cancers.
Daiichi Sankyo (DSKYF)
 Image via Wikipedia
Image via WikipediaPlexxikon is sitting pretty right now, largely due to the stellar performance of its lead experimental drug, code named PLX4032. After the Berkeley, CA-based firm and its partner Roche reported exciting data from trials of the drug in patients with melanoma over the past year, Japan's Daiichi Sankyo swooped in and bought Plexxikon in a $935 million deal revealed in February. What's more, Plexxikon CEO Peter Hirth told FierceBiotech that the company's Japanese owner plans to give the small drug developer a degree of autonomy and funding to continue its impressive work.
Plexxikon reported in January that melanoma patients on the drug in a Phase III trial were showing better overall survival and on average were living longer without their cancer progressing than those who were on the standard therapy for melanoma, dacarbazine. The drug is targeted for patients with a BFAF mutation. Roche is developing a DNA-based test to identify patients with the BRAF mutation, which causes unchecked cell growth and cancer. In general, BRAF mutations show up in most melanomas.
Plexxikon, a 2010 Fierce 15 company, published Phase I data in an August 2010 issue of the New England Journal of Medicine. The article said that 81 percent of melanoma patients on the drug had their tumors shrink by at least 30 percent.
Ariad Pharmaceuticals (ARIA)
 Image via Wikipedia
Image via WikipediaThe big story at Cambridge, MA-based Ariad Pharmaceuticals ($ARIA) is its leukemia drug ponatinib, despite the fact that its lead experimental cancer drug is ridaforolimus (which is advancing in partnership with Merck). Ponatinib is now in a pivotal Phase II clinical trial as a treatment for patients with chronic myeloid leukemia or Philadelphia positive acute lymphoblastic leukemia whose cancers are resistant to Bristol-Myers Squibb's Sprycel or Novartis's Tasigna.
Ponatinib is designed to target multiple mutant forms of BCR-ABL fusion proteins, making it a potentially potent next-generation inhibitor of tyrosine kinases. In December the company reported that in a Phase I trial 66 percent of patients on the drug showed a major cytogenetic response, evidence that the drug was weakening their cancer (and such responses were shown in 100 percent of patients with a T315I mutation). The firm is interested in partnering on the development of ponatinib in certain markets outside of the U.S; so another pharma deal might be in the offing for the company.
Seattle Genetics (SGEN) Takeda Pharmaceuticals (TKPHF)
After promising results in clinical trials revealed late last year, SGN-35 (brentuximab vedotin) has a shot at being approved later this year for immune system cancers known as Hodgkin's lymphoma and anaplastic large cell lymphomas that have been resistant to prior therapies or come back after a period of time.
Seattle Genetics ($SGEN) and Millennium: The Takeda Oncology Company reported in December that the drug--which is an anti-CD30 monoclonal antibody linked to cancer-killing agent called an auristatin--completely wiped out visible signs of disease in just more than half of the 58 patients with forms of anaplastic large cell lymphomas in a Phase II study, with an additional third of the patients' tumors shrinking by at least half.
In a pivotal trial involving 102 patients with relapsed or resistant Hodgkin's lymphoma, the drug rendered the cancer undetectable in 34 percent of patients and provided partial remissions for 40 percent of volunteers in the trial. Seattle Genetics has U.S. and Canadian marketing rights to the drug, and Takeda controls commercialization of the treatment in the rest of the world.
Aveo Pharmaceuticals (AVEO)
 Image via CrunchBase
Image via CrunchBaseTuan Ha-Ngoc, the president and CEO of Aveo Pharmaceuticals ($AVEO), is leading a very smart charge to advance tivozanib to the market for kidney cancers called advanced renal cell carcinoma (RCC).
In June 2010, the company showed results from a Phase II study of a the drug--a potent blocker of all three vascular endothelial growth factor (VEGF) receptors, which play a role in the formation of blood vessels that feed tumors--that patients taking the drug on average lived without their cancer getting worse for 11.8 months. That same key indicator of effectiveness was nearly 14.8 months among patients with a clear cell form of RCC who had prior surgeries to remove at least part of their kidneys.
In the company's 517-patient Phase III trial comparing tivozanib head-to-head with Onyx Pharmaceuticals' and Bayer's Nexavar, patients who enter the study must have a clear cell form of the disease and previously have had the surgery to be eligible (the types of patients who did really well in the Phase II study). Aveo plans to report results of the trial in mid-2011. It's also worth keeping an eye on Pfizer's similar drug, axitinib, for which the drug giant plans to submit in 2011 applications for U.S. and European regulatory approvals as a treatment for patients with kidney cancer.
Aveo is sharing equally North American and European development costs and profits related to tivozanib with Japan's Astellas Pharma. Kyowa Hakko Kirin has rights to develop and commercialize the drug in Asia.
ImmunoGen (IMGN)
Here's more evidence that linking targeting antibodies with cancer-killing drugs can pack a powerful one-two punch against tumors. This "armed antibody" drug due to be submitted (again) for regulatory approval in mid-2012. T-DM1 has been shining in clinical trials involving women with breast cancer for years, but the FDA handed back Swiss drug giant Roche's application for approval of the drug in August 2010. That application was made with promising Phase II data on the treatment, but regulators apparently want to see results from larger studies underway.
\T-DM1 is truly a two-pronged weapon against breast tumors. It uses linker technology from Waltham, MA-based ImmunoGen ($IMGN) to equip Roche's Herceptin, an antibody that binds to HER2-expressing breast tumors, with a cell-killing agent known as DM1. This enables the antibody to home in on the cancer target and deliver the cell-killing agent into tumor cells.
Preliminary data released in October from a 137-patient Phase II study showed that 47.8 percent of breast cancer patients on T-DM1 had a confirmed objective response on T-DM1, compared with 41.4 percent who were taking a combination of Herceptin and a chemotherapy drug, taxane.
Related articles
- Analyst bets on approval of Pfizer's lung cancer drug (fiercebiotech.com)
- Blocking molecular target could make more cancers treatable with PARP inhibitors (medicalxpress.com)
- Pfizer Lung Cancer Drug to Get Priority Review (xconomy.com)
- ASCO highlights targeted cancer drugs from Pfizer, Exelixis (fiercebiotech.com)
- Globe highlights targeted cancer drugs from Pfizer, Roche ahead of ASCO (fiercebiotech.com)
- Fda Votes 6-0 Against Cancer Drug Avastin; Why? (brittaj17.wordpress.com)
- Roche preps vismodegib app with "profound" efficacy data (fiercebiotech.com)
- Chemo for Late-Stage Cancer Patients May Be Unjustified (nlm.nih.gov)
- Study: FDA beats EMA on new cancer drug approvals (fiercebiotech.com)
- ONYX Pharmaceuticals Shares Popped: What You Need to Know (fool.com)

60 Minutes on J. Craig Venter: Designing Life
To contact us Click HERE
 Image via WikipediaJ. Craig Venter: Designing Life
Image via WikipediaJ. Craig Venter: Designing Life
June 12, 2011 5:00 PM
Steve Kroft profiles famous microbiologist J. Craig Venter, whose scientists have already mapped the human genome and created what he calls "the first synthetic species."
Read more: http://www.cbsnews.com/video/watch/?id=7369820n&tag=cbsnewsMainColumnArea.8#ixzz1RZ052a3e
(CBS News)
This story was first published on Nov. 21, 2010. It was updated on June 12, 2011.
For generations, scientists have wrestled with the idea of creating new forms of life in the laboratory. Now that age is upon us.
Watch the Segment »
 Image by PhOtOnQuAnTiQuE via Flickr
Image by PhOtOnQuAnTiQuE via Flickr
He is one of the most famous scientists in the world, known for his pioneering work in deciphering the human genetic code. But as we reported last fall, he is also one of the most controversial - an iconoclast with a brilliant mind and an outsized ego who has flaunted the conventional wisdom, and tweaked the staid scientific establishment at every turn.
You don't have to spend much time with Venter to understand that he likes to go fast, as "60 Minutes" correspondent Steve Kroft found out first-hand when the scientist took Kroft for a spin in his Aston Martin.
Venter is an adrenaline junky, whose willingness to take big risks has led to bold scientific breakthroughs. And he is not exactly shy about touting those achievements.
Asked where he would rank himself in terms of scientific accomplishments, Venter told Kroft, "Well, in the field of genomics, I think the record is pretty clear cut: the first genome in history, the first draft of the human genome, the first complete version of the human genome. And having the first synthetic cells."
"So, the answer to the question is pretty high?" Kroft asked.
 Image by Wombatunderground1 via Flickr
Image by Wombatunderground1 via Flickr
Extra: Exploring the oceans
Extra: The first "synthetic cell"
Extra: Human genome & disappointment
"I mean it's really hard to assess that yourself. But I think the teams that we have, and what we've accomplished are certainly amongst the biggest discoveries in modern science," Venter said.
If you have some stereotype of a scientist in your mind, Venter probably doesn't fit it. He has scuba-dived with sharks to gather microbes in the Pacific, and spent much of the past summer sailing through the Greek isles on his 95-foot research vessel, plucking new genetic material from the sea. He rarely goes anywhere without his wife, Heather, and their dog Darwin. And their home high above the Pacific in La Jolla, Calif., suggests the quest for scientific truth requires no vow of poverty.
"I have been lucky," Venter acknowledged. "Sort of the accidental millionaire in terms of people keep giving me money to start companies to exploit the science."
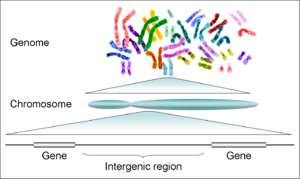 Image via Wikipedia
Image via Wikipedia
He runs both a privately-held biotech company called Synthetic Genomics, and a non-profit research lab, the J. Craig Venter Institute. Together, they employ more than 500 people on two coasts, including one Nobel laureate, Hamilton Smith, and some of the top scientists in the world.
"I'm much more like an orchestra conductor than the violinist," Venter said.
When asked what he thinks his greatest talent is, Venter said, "I have an unusual type of thinking. I have no visual memory whatsoever. Everything is conceptual to me. So I think that's part of it. I see things differently."
Venter likes to think big, and his latest advancement is no exception. He removed a Petri dish from an incubator in his lab and held it up to the light, revealing small dark specks of bacteria.
 Image by elizaIO via Flickr"This is the first synthetic species," Venter told Kroft.
Image by elizaIO via Flickr"This is the first synthetic species," Venter told Kroft.
"And how long did it take you to make this?" Kroft asked.
"Well, if you count the total time from the conception, about 15 years," Venter replied.
The project cost about $40 million over that time period, Venter told Kroft.
In practical terms, what Venter's team has created is about as useful as the mold that grows in a bachelor's refrigerator, but scientifically it is a milestone. The bacteria, which is similar to one found in the intestines of goats, was designed on a computer, manufactured in the laboratory, and gets its genetic instructions from a synthetic chromosome made by man, not nature.
"It's alive and self-replicating. That means it can indefinitely grow and make copies of itself," Venter explained.
Asked if he designed it to do anything in particular, Venter said, "No. We designed this just to see if we could do this whole experiment using synthetic DNA. And now that we know we can do it, it's worth the effort to now make the things that could be valuable."
 Image via WikipediaJust how valuable remains to be seen, but Venter believes this is the first baby step in a biological revolution: one, in which it will be possible to custom design and re-program bacteria and other organisms to churn out new medicines, foods, and clean sources of energy.
Image via WikipediaJust how valuable remains to be seen, but Venter believes this is the first baby step in a biological revolution: one, in which it will be possible to custom design and re-program bacteria and other organisms to churn out new medicines, foods, and clean sources of energy.
"What you're doing is programming cells like somebody would program software," Kroft remarked.
"DNA is the software of life. There's no question about it," Venter replied. "And the key to evolution of life on this planet and now to the key to the future of life on this planet is understanding how to write that software."
"So, you see bioengineered fuel for example?" Kroft asked.
"I see in the future, bioengineered almost everything you can imagine that we use," Venter said.
 Image via Wikipedia
Image via Wikipedia
Asked how far off some of this is, Venter said, "The first things will start to come out in the next few years. I think possibly next year's flu vaccine could come from these synthetic DNA processes. Instead of months to make a new vaccine each year, we could do it in 24 hours or less."
He has already signed a contract with a major pharmaceutical firm to try and do it. BP is funding research to experiment with underground microbes that feed off coal and produce natural gas. And Exxon Mobil has committed $300 million to Venter's company to genetically enhance an algae that lives off carbon dioxide and produces an oil that can be refined into gasoline.
"So you're trying to cut down on CO2 in the atmosphere, which people believe causes global warming and also create a fuel?" Kroft asked, while touring Venter's greenhouse, which is filled with bags of algae under study.
"The question is on the scale that it needs to be done at. You know? Facilities the size of San Francisco," Venter said.
 Image via Wikipedia
Image via Wikipedia
Venter and his team are not the only players in this growing field known as "synthetic biology." For years, DuPont has been using genetically modified bacteria to make a compound used in clothing and carpets; Amyris discovered a way to genetically modify yeast to produce an anti-malarial drug; and another company, LS9, has altered the genes of E. coli bacteria to produce fuel. But all of these companies are modifying a few genes, not designing all of them.
Venter's rivals say his method is commercially impractical. But he has made a career out of bucking the scientific establishment, and earned lots of enemies with his brash behavior and his knack for grabbing research money and the spotlight.
When Kroft asked Venter what his faults are, he replied, "Probably impatience is the biggest one. I don't suffer fools too well. I'm not going to ever win a political contest."
"A lot of people have said you're a self promoter, an egomaniac?" Kroft asked. "True? Partially true? Not true at all?"
"You know, if we hold a press conference it's considered self-promotion," Venter said. "But, somebody at a university, the university holds the press conference, and that's not self promotion."
"Overly ambitious?" Kroft asked.
"I'm sure I'm very guilty of that," Venter replied.
That wasn't always the case. He grew up in the suburbs of San Francisco as the prototypical surfer dude and a classic underachiever.
 Image by jurvetson via Flickr
Image by jurvetson via Flickr
"I was a horrible student. I really hated school," he remembered.
Asked if he was good in math and science, Venter said, "I was not really good in anything, you know? I almost flunked out of high school."
He did get a college scholarship for swimming. "But I didn't take it," Venter said. "So at age 17, I moved to southern California to take up surfing."
In 1965 reality set in. He got drafted off his surfboard, joined the Navy as a medic, and was sent to Vietnam to work at a field hospital in Da Nang. The experience changed his life and motivated him to go back to school and pursue a career in medical research.
 Image via Wikipedia
Image via Wikipedia
He became a rising star at the National Institutes of Health, and just as quickly grew frustrated with the politics and bureaucracy of government science. When the NIH declined to fund some of his unorthodox new ideas, he left and found private investors who would.
"I think we have a real problem with how science is funded and done in this country," Venter said. "I mean almost every breakthrough I've been associated with is from having independent money. And once they worked, we can get tons of government money to follow up on it. But, we could never get the money to do the initial experiment."
In 1998, a company that made cutting edge technology to analyze DNA hired him to take on the federal government in a race to identify all the genetic material in the human body. The federally funded human genome project had already been working on it for years.
Asked why he decided to challenge the government, Venter said, "The way it was being done just didn't make any sense. We ended up doing it in nine months instead of 15 years. That's a big difference."
When the competition produced bad blood and bad publicity in the scientific community, the Clinton administration arranged for the two sides to announce a truce and a tie, even though many believe Venter's company, Celera Genomics, was ahead. But for Venter the celebration was short-lived: the tension between making science and making money and personality conflicts with his corporate bosses got Venter sacked a year and a half later.
"You accomplished all this stuff. And you got fired by the company that brought you in to do this," Kroft remarked. "They locked the doors."
"They locked the doors and sent me away," Venter acknowledged.
The experience left him deeply depressed, but he was financially well off and still in business, having endowed his research institute with $100 million in stock at the height of biotech boom. Within a few years, he was once again making waves in the world of science.
Only this time, at age 64, he's not just trying to decipher genetic codes. Now, he's trying to create them.
"This is a quote from one of your critics: 'He's trying to short-circuit millions of years of evolution and create his own version of a second genesis. It's the height of hubris. It's irresponsible. And he can't tell you it's going to be safe,'" Kroft said.
"Except for the second part, I was taking that as a compliment," Venter replied, laughing. "I can tell you what we're doing is safe. There's no way that I can guarantee that other people that use these tools will do intelligent, safe experiments with it. But I think the chance of evil happening with this and somebody even trying to do deliberate evil would be pretty hard."
"Why?" Kroft asked.
"Because the complexity of biology," Venter said. "You know, we're not working with human pathogens.
We're working with algae cells. And part of our design is cells that won't survive outside of a facility or a laboratory. And we think other scientists will adopt these same approaches."
"There are some things that concern you about this?" Kroft asked.
"Well, it is powerful technology. It's something that needs to be monitored absolutely," Venter replied.
President Obama was concerned enough to ask his commission on bioethics to hold hearings on Venter's new technology shortly after the results were published in the journal Science. Apart from the legal and regulatory questions raised, there are some moral and ethical ones as well.
"There are a lot of people in this country who don't think that you ought to screw around with nature," Kroft remarked.
"We don't have too many choices now. We are a society that is one hundred percent dependent on science.
We're going to go up in our population in the next 40 years; we can't deal with the population we have without destroying our environment," Venter said.
"But aren't you playing God?" Kroft asked.
"We're not playing anything. We're understanding the rules of life," Venter said.
"But that's more than studying life, that's changing life," Kroft pointed out.
 Image by abekat via Flickr
Image by abekat via Flickr
"Well, domesticating animals was changing life, domesticating corn. When you do cross-breeding of plants, you're doing this blind experiment where you're just mixing DNA of different types of cells and just seeing what comes out of it," Venter said.
"This is a little different though, this is another step, isn't it?" Kroft asked.
"Yeah, now we're doing it in a deliberate design fashion with tiny bacteria. I think it's much healthier to do it based on some knowledge and a better understanding of life than to do it blindly and randomly," Venter said.
"You know, I've asked two or three times, 'Do you think you're playing God?' I mean, do you believe in God?" Kroft asked.
"No," Venter replied. "I believe the universe is far more wonderful than just assuming it was made by some higher power. I think the fact that these cells are software-driven machines and that software is DNA and that truly the secret of life is writing software, is pretty miraculous. Just seeing that process in the simplest forms that we're just witnessing is pretty stunning."

 Image via WikipediaJ. Craig Venter: Designing Life
Image via WikipediaJ. Craig Venter: Designing LifeJune 12, 2011 5:00 PM
Steve Kroft profiles famous microbiologist J. Craig Venter, whose scientists have already mapped the human genome and created what he calls "the first synthetic species."
Read more: http://www.cbsnews.com/video/watch/?id=7369820n&tag=cbsnewsMainColumnArea.8#ixzz1RZ052a3e
(CBS News)
This story was first published on Nov. 21, 2010. It was updated on June 12, 2011.
For generations, scientists have wrestled with the idea of creating new forms of life in the laboratory. Now that age is upon us.
Watch the Segment »
 Image by PhOtOnQuAnTiQuE via Flickr
Image by PhOtOnQuAnTiQuE via Flickr Web Extras
- J. Craig Venter: Designing Life
- Extra: Developing the first "synthetic cell"
- Extra: Exploring the oceans
- Extra: The human genome & disappointment
- More »
He is one of the most famous scientists in the world, known for his pioneering work in deciphering the human genetic code. But as we reported last fall, he is also one of the most controversial - an iconoclast with a brilliant mind and an outsized ego who has flaunted the conventional wisdom, and tweaked the staid scientific establishment at every turn.
You don't have to spend much time with Venter to understand that he likes to go fast, as "60 Minutes" correspondent Steve Kroft found out first-hand when the scientist took Kroft for a spin in his Aston Martin.
Venter is an adrenaline junky, whose willingness to take big risks has led to bold scientific breakthroughs. And he is not exactly shy about touting those achievements.
Asked where he would rank himself in terms of scientific accomplishments, Venter told Kroft, "Well, in the field of genomics, I think the record is pretty clear cut: the first genome in history, the first draft of the human genome, the first complete version of the human genome. And having the first synthetic cells."
"So, the answer to the question is pretty high?" Kroft asked.
 Image by Wombatunderground1 via Flickr
Image by Wombatunderground1 via Flickr Extra: Exploring the oceans
Extra: The first "synthetic cell"
Extra: Human genome & disappointment
"I mean it's really hard to assess that yourself. But I think the teams that we have, and what we've accomplished are certainly amongst the biggest discoveries in modern science," Venter said.
If you have some stereotype of a scientist in your mind, Venter probably doesn't fit it. He has scuba-dived with sharks to gather microbes in the Pacific, and spent much of the past summer sailing through the Greek isles on his 95-foot research vessel, plucking new genetic material from the sea. He rarely goes anywhere without his wife, Heather, and their dog Darwin. And their home high above the Pacific in La Jolla, Calif., suggests the quest for scientific truth requires no vow of poverty.
"I have been lucky," Venter acknowledged. "Sort of the accidental millionaire in terms of people keep giving me money to start companies to exploit the science."
 Image via Wikipedia
Image via WikipediaHe runs both a privately-held biotech company called Synthetic Genomics, and a non-profit research lab, the J. Craig Venter Institute. Together, they employ more than 500 people on two coasts, including one Nobel laureate, Hamilton Smith, and some of the top scientists in the world.
"I'm much more like an orchestra conductor than the violinist," Venter said.
When asked what he thinks his greatest talent is, Venter said, "I have an unusual type of thinking. I have no visual memory whatsoever. Everything is conceptual to me. So I think that's part of it. I see things differently."
Venter likes to think big, and his latest advancement is no exception. He removed a Petri dish from an incubator in his lab and held it up to the light, revealing small dark specks of bacteria.
 Image by elizaIO via Flickr"This is the first synthetic species," Venter told Kroft.
Image by elizaIO via Flickr"This is the first synthetic species," Venter told Kroft."And how long did it take you to make this?" Kroft asked.
"Well, if you count the total time from the conception, about 15 years," Venter replied.
The project cost about $40 million over that time period, Venter told Kroft.
In practical terms, what Venter's team has created is about as useful as the mold that grows in a bachelor's refrigerator, but scientifically it is a milestone. The bacteria, which is similar to one found in the intestines of goats, was designed on a computer, manufactured in the laboratory, and gets its genetic instructions from a synthetic chromosome made by man, not nature.
"It's alive and self-replicating. That means it can indefinitely grow and make copies of itself," Venter explained.
Asked if he designed it to do anything in particular, Venter said, "No. We designed this just to see if we could do this whole experiment using synthetic DNA. And now that we know we can do it, it's worth the effort to now make the things that could be valuable."
 Image via WikipediaJust how valuable remains to be seen, but Venter believes this is the first baby step in a biological revolution: one, in which it will be possible to custom design and re-program bacteria and other organisms to churn out new medicines, foods, and clean sources of energy.
Image via WikipediaJust how valuable remains to be seen, but Venter believes this is the first baby step in a biological revolution: one, in which it will be possible to custom design and re-program bacteria and other organisms to churn out new medicines, foods, and clean sources of energy."What you're doing is programming cells like somebody would program software," Kroft remarked.
"DNA is the software of life. There's no question about it," Venter replied. "And the key to evolution of life on this planet and now to the key to the future of life on this planet is understanding how to write that software."
"So, you see bioengineered fuel for example?" Kroft asked.
"I see in the future, bioengineered almost everything you can imagine that we use," Venter said.
 Image via Wikipedia
Image via WikipediaAsked how far off some of this is, Venter said, "The first things will start to come out in the next few years. I think possibly next year's flu vaccine could come from these synthetic DNA processes. Instead of months to make a new vaccine each year, we could do it in 24 hours or less."
He has already signed a contract with a major pharmaceutical firm to try and do it. BP is funding research to experiment with underground microbes that feed off coal and produce natural gas. And Exxon Mobil has committed $300 million to Venter's company to genetically enhance an algae that lives off carbon dioxide and produces an oil that can be refined into gasoline.
"So you're trying to cut down on CO2 in the atmosphere, which people believe causes global warming and also create a fuel?" Kroft asked, while touring Venter's greenhouse, which is filled with bags of algae under study.
"The question is on the scale that it needs to be done at. You know? Facilities the size of San Francisco," Venter said.
 Image via Wikipedia
Image via WikipediaVenter and his team are not the only players in this growing field known as "synthetic biology." For years, DuPont has been using genetically modified bacteria to make a compound used in clothing and carpets; Amyris discovered a way to genetically modify yeast to produce an anti-malarial drug; and another company, LS9, has altered the genes of E. coli bacteria to produce fuel. But all of these companies are modifying a few genes, not designing all of them.
Venter's rivals say his method is commercially impractical. But he has made a career out of bucking the scientific establishment, and earned lots of enemies with his brash behavior and his knack for grabbing research money and the spotlight.
When Kroft asked Venter what his faults are, he replied, "Probably impatience is the biggest one. I don't suffer fools too well. I'm not going to ever win a political contest."
"A lot of people have said you're a self promoter, an egomaniac?" Kroft asked. "True? Partially true? Not true at all?"
"You know, if we hold a press conference it's considered self-promotion," Venter said. "But, somebody at a university, the university holds the press conference, and that's not self promotion."
"Overly ambitious?" Kroft asked.
"I'm sure I'm very guilty of that," Venter replied.
That wasn't always the case. He grew up in the suburbs of San Francisco as the prototypical surfer dude and a classic underachiever.
 Image by jurvetson via Flickr
Image by jurvetson via Flickr"I was a horrible student. I really hated school," he remembered.
Asked if he was good in math and science, Venter said, "I was not really good in anything, you know? I almost flunked out of high school."
He did get a college scholarship for swimming. "But I didn't take it," Venter said. "So at age 17, I moved to southern California to take up surfing."
In 1965 reality set in. He got drafted off his surfboard, joined the Navy as a medic, and was sent to Vietnam to work at a field hospital in Da Nang. The experience changed his life and motivated him to go back to school and pursue a career in medical research.
 Image via Wikipedia
Image via WikipediaHe became a rising star at the National Institutes of Health, and just as quickly grew frustrated with the politics and bureaucracy of government science. When the NIH declined to fund some of his unorthodox new ideas, he left and found private investors who would.
"I think we have a real problem with how science is funded and done in this country," Venter said. "I mean almost every breakthrough I've been associated with is from having independent money. And once they worked, we can get tons of government money to follow up on it. But, we could never get the money to do the initial experiment."
In 1998, a company that made cutting edge technology to analyze DNA hired him to take on the federal government in a race to identify all the genetic material in the human body. The federally funded human genome project had already been working on it for years.
Asked why he decided to challenge the government, Venter said, "The way it was being done just didn't make any sense. We ended up doing it in nine months instead of 15 years. That's a big difference."
When the competition produced bad blood and bad publicity in the scientific community, the Clinton administration arranged for the two sides to announce a truce and a tie, even though many believe Venter's company, Celera Genomics, was ahead. But for Venter the celebration was short-lived: the tension between making science and making money and personality conflicts with his corporate bosses got Venter sacked a year and a half later.
"You accomplished all this stuff. And you got fired by the company that brought you in to do this," Kroft remarked. "They locked the doors."
"They locked the doors and sent me away," Venter acknowledged.
The experience left him deeply depressed, but he was financially well off and still in business, having endowed his research institute with $100 million in stock at the height of biotech boom. Within a few years, he was once again making waves in the world of science.
Only this time, at age 64, he's not just trying to decipher genetic codes. Now, he's trying to create them.
"This is a quote from one of your critics: 'He's trying to short-circuit millions of years of evolution and create his own version of a second genesis. It's the height of hubris. It's irresponsible. And he can't tell you it's going to be safe,'" Kroft said.
"Except for the second part, I was taking that as a compliment," Venter replied, laughing. "I can tell you what we're doing is safe. There's no way that I can guarantee that other people that use these tools will do intelligent, safe experiments with it. But I think the chance of evil happening with this and somebody even trying to do deliberate evil would be pretty hard."
"Why?" Kroft asked.
"Because the complexity of biology," Venter said. "You know, we're not working with human pathogens.
We're working with algae cells. And part of our design is cells that won't survive outside of a facility or a laboratory. And we think other scientists will adopt these same approaches."
"There are some things that concern you about this?" Kroft asked.
"Well, it is powerful technology. It's something that needs to be monitored absolutely," Venter replied.
President Obama was concerned enough to ask his commission on bioethics to hold hearings on Venter's new technology shortly after the results were published in the journal Science. Apart from the legal and regulatory questions raised, there are some moral and ethical ones as well.
"There are a lot of people in this country who don't think that you ought to screw around with nature," Kroft remarked.
"We don't have too many choices now. We are a society that is one hundred percent dependent on science.
We're going to go up in our population in the next 40 years; we can't deal with the population we have without destroying our environment," Venter said.
"But aren't you playing God?" Kroft asked.
"We're not playing anything. We're understanding the rules of life," Venter said.
"But that's more than studying life, that's changing life," Kroft pointed out.
 Image by abekat via Flickr
Image by abekat via Flickr"Well, domesticating animals was changing life, domesticating corn. When you do cross-breeding of plants, you're doing this blind experiment where you're just mixing DNA of different types of cells and just seeing what comes out of it," Venter said.
"This is a little different though, this is another step, isn't it?" Kroft asked.
"Yeah, now we're doing it in a deliberate design fashion with tiny bacteria. I think it's much healthier to do it based on some knowledge and a better understanding of life than to do it blindly and randomly," Venter said.
"You know, I've asked two or three times, 'Do you think you're playing God?' I mean, do you believe in God?" Kroft asked.
"No," Venter replied. "I believe the universe is far more wonderful than just assuming it was made by some higher power. I think the fact that these cells are software-driven machines and that software is DNA and that truly the secret of life is writing software, is pretty miraculous. Just seeing that process in the simplest forms that we're just witnessing is pretty stunning."
Related articles
- Genomic research: Faster, cheaper, more hope (boingboing.net)
- What's the Future of Synthetic Biology? (technologyreview.com)
- My Genome: My Life,a life decoded(autobiography by J. Craig Venter) (zemali.wordpress.com)
- Designing life: What's next for J. Craig Venter? (cbsnews.com)

Kaydol:
Kayıtlar (Atom)